ASCO 2023 – Pfizer Pushes Forward In Drug-Resistant Breast Cancer
Executive Summary
While Ibrance has been bested by class rivals Pfizer is working on projects that might overcome CDK4/6 inhibitor resistance, although it is not alone.
CDK4/6 inhibitors – Eli Lilly and Company’s Verzenio, Novartis AG’s Kisqali and Pfizer Inc.’s Ibrance – have proven efficacy in ER-positive/Her2-negative breast cancer, but a significant proportion of patients still relapse. CDK2 is receiving increasing attention as a possible answer to resistance to these drugs, and the American Society of Clinical Oncology (ASCO) annual meeting saw a couple of early data sets on some of the most advanced assets.
Pfizer and Blueprint Bio Inc. presented posters on their respective agents at the meeting, with projects from Incyte Corporation, Cyclacel Pharmaceuticals, Inc. and a couple of private biotechs also in the clinic. Work is still very early and, though some encouraging signals can be seen, it is unclear whether CDK2 inhibition is a viable monotherapy mechanism.
Upregulation of cyclin E1 and the subsequent activation of CDK2 has been shown to promote resistance to CDK4/6 inhibitors. And there is another angle here: some tumours, particularly ovarian and breast, overexpress cyclin E and/or harbour cyclin E1 (CCNE1) gene amplifications.
As such, several trials of CDK2-targeted agents are also recruiting patients with CCNE1-amplified tumours.
|
Hitting CDK2: the pipeline |
|||
|
Project |
Mechanism |
Company |
Status |
|
PF-07104091 |
CDK2 inhibitor |
Pfizer |
Ph1/2 trial in small cell lung, breast and ovarian cancers; also in combo trial with CDK4 inhibitor PF-07220060 |
|
BLU-222 |
CDK2 inhibitor |
Blueprint Medicines |
|
|
INCB0123667 |
CDK2 inhibitor |
Incyte |
|
|
INX-315 |
CDK2 inhibitor |
Incyclix |
Ph1/2 trial in CDK4/6 refractory and CCNE1-amplified tumours |
|
ARTS-021 |
CDK2 inhibitor |
||
|
Fadraciclib (CYC065) |
CDK2/9 inhibitor |
Cyclacel |
|
|
Source: Evaluate Pharma & clinicaltrials.gov. |
|||
Asco saw early results from Pfizer’s PF-07104091, which is probably the most advanced CDK2 asset, from a solid tumour study that enriched for CDK4/6 inhibitor-resistant metastatic breast cancer patients.
Of 16 efficacy-evaluate mBC patients, three partial responses were seen (19%). Two had a duration of response that stretched beyond six months; all patients were heavily pretreated. The maximum tolerated dose has been reached, and the study is now proceeding into expansion cohorts that comprise breast cancer patients who will also be given endocrine therapy, and a monotherapy ovarian cancer cohort.
A separate poster on Blueprint’s BLU-222 in the Vela trial saw no such activity, with only one partial response among 27 treated patients. The company stressed that the patient had previously received five lines of therapy, including Ibrance and Verzenio, and added that the maximum tolerated dose had not been reached.
The Vela trial is continuing with Kisqali and fulvestrant combination arms, but the lack of monotherapy activity here could be considered concerning.
Blueprint told Evaluate Vantage that it believes combinations will be needed, adding that monotherapy may have a role in CCNE1-amplified cancers.
Notably, Pfizer has already initiated a separate combination trial of PF-07104091 with its selective CDK4 inhibitor PF-07220060. This could be read as a safety net in case CDK2 inhibition is not a sufficiently potent lone actor in the treatment-resistant setting, although the developer probably has bigger hopes here.
PF-07220060 was the subject of its own poster, concerning early data from a similar solid tumour trial enriched for breast cancer patients failed by CDK4/6 inhibition. Once again, lack of single-agent activity was seen, with 18 cases of stable disease being the best responses from 29 evaluable patients.
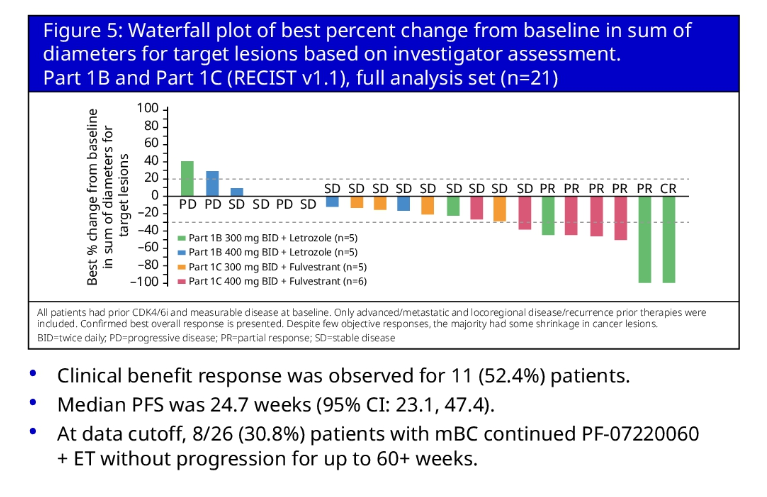
A 21-patient hormone therapy combo arm fared better, with one complete response and five partial responses. Memorial Sloan Kettering’s Dr Komal Jhaveri, who discussed the two posters for ASCO, pointed out that PF-07220060 seemed to have less myelotoxicity than approved CDK4/6 inhibitors.
PF-07220060 + endocrine therapy (NCT04557449)
Pfizer is already pushing on with PF-07220060 in expansion cohorts in resistant and CDK4/6-naïve patients. This strategy makes sense, given that it is now appreciated that CDK4 plays a more important role than CDK6 in breast cancer.
Indeed, it is thought this explains Ibrance’s seemingly poorer efficacy versus Verzenio and Kisqali: the Pfizer drug has the least selectively of these agents for CDK4 versus the CDK6 subtype.
After missing the goal first time around with cell cycle regulation and CDK inhibition Pfizer seems to be trying again. Dr Timothy Yap, lead author on both the CDK2 and CDK4 posters, said high selectivity is the "secret sauce" of these two agents.
– Amy Brown ([email protected])
This article originally appeared in Evaluate Vantage. Evaluate Vantage and Scrip are part of the same parent company, Norstella.