ASCO 2023 – Bicara Eyes Head And Neck Niche
Executive Summary
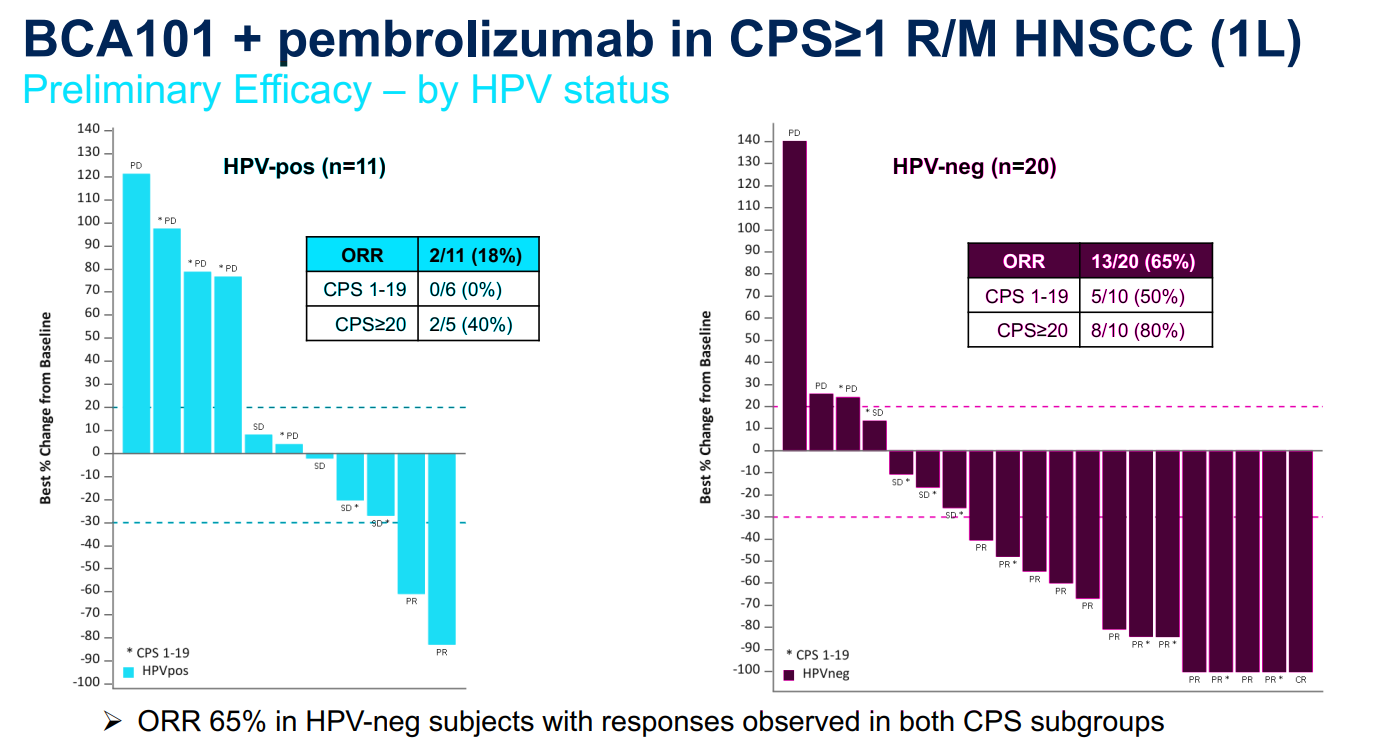
Impressive data in HPV-negative disease, potentially beating Keytruda, has the private group making pivotal plans.
Bicara Therapeutics raised $108m in March on the back of encouraging early data on BCA101, a bispecific antibody that hits both EGFR and TGF-beta. With strong responses now confirmed in head and neck cancer, and a registrational trial potentially on the horizon, the group is already thinking about its next financing.
A 48% overall response rate in 31 patients was presented on 5 June at the American Society of Clinical Oncology annual meeting, from an ongoing phase 1/1b study of BCA101 plus Keytruda in recurrent/metastatic disease. The result was driven by a remarkable 65% response rate, including one complete remission, in a cohort of patients whose cancer had no human papillomavirus involvement; this tumour type typically responds poorly to monotherapy treatment with the Merck & Co., Inc. checkpoint inhibitor.
A first-line randomised trial in HPV-negative patients will be the next step, chief executive Claire Mazumdar tells Evaluate Vantage. FDA feedback is awaited, but there is a strong possibility that the trial will be registrational, she says, and that will require a further cash injection to complete.
“Any registrational trial would need to be powered on overall survival so it would have to be large,” she said, speaking at ASCO in Chicago. “If we’re going to start a study like that we want to be fully funded.”
Standard of care
Keytruda is standard of care in head and neck cancer; the anti-PD-1 MAb won approval in a first-line setting here on the back of the Keynote-048 trial. In patients with PD-L1 expression ≥1 – the group that Bicara is eyeing – ORR hit 19%.
Mazumdar says Merck has never published data split by HPV status, but it is understood that the response rate in HPV-negatives sits around 15-19%, with positives in the 19-25% range.
HPV-negative head and neck cancer tends to be driven by use of chewing tobacco or smoking, and as such is frequently driven by EGFR mutations. This makes the tumours more resistant versus HPV-positive disease, Mazumdar says.
Source: Dr Glenn J Hanna and Asco.
There is a mechanistic reason why BCA101 is working so well in these patients, she says, through both EGFR inhibition and the disabling of TGF-beta. Mazumdar says Bicara has evidence that the agent is preventing epithelial-mesenchymal transition – a precursor to metastasis.
“That’s what’s helping us see deep responses – the tumour microenvironment is being remodelled,” she says. “Our median progression-free survival of 6.6 months is more than double Keytruda [monotherapy]. We hope to update that later this year at another conference.”
In Keynote-048 Keytruda monotherapy generated mPFS of 3.2 months and significantly extended overall survival by two months over chemotherapy (12.3 versus 10.3 months, HR 0.78, p=0.0171). This provides the bar for Bicara to beat, though safety is also something to watch.
In the front-line Keytruda trial 12% of patients discontinued, mostly owing to sepsis or pneumonia. Skin toxicities are BCA101’s biggest side effect detected so far, and although only three patients (9%) discontinued the phase 1 trial, one of these was down to grade 3 drug-related tracheal haemorrhage.
TGF-beta has long been considered a promising target for anticancer agents, but several projects have disappointed, Merck KGaA and GSK’s bintrafusp alfa being probably the most high-profile failure.
“Prior assets have not been able to achieve enough inhibition [of TGF-beta] at the tumour. We were able to show very early on dose escalation that we had tumour engagement,” Mazumdar says.
The larger trial needed to prove all this will hopefully start next year.
– Amy Brown ([email protected])
This article originally appeared in Evaluate Vantage. Evaluate Vantage and Scrip are part of the same parent company, Norstella.