
PDUFA VI: A Machine To Speed R&D Time, Cut Costs
The biopharmaceutical industry is hoping the terms set under the negotiations with the FDA for the sixth round of the Prescription Drug User Fee Act will help reduce the time and money spent on developing medicines, allowing companies to get their products into the commercial setting quicker and maximize those therapies' potential.
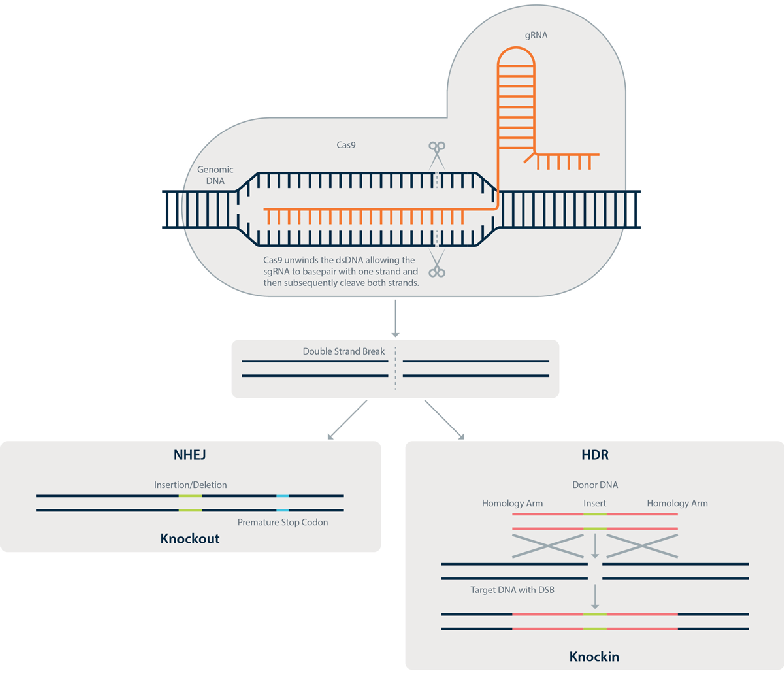
China Surprises With First CRISPR Trial Despite Regulatory Lag, Concerns
The lack of a comprehensive regulatory pathway has not hindered Chinese researchers from forging ahead with the world's first human clinical study using CRISPR-Cas9 gene editing technology, which will be used to treat advanced lung cancer.

ABPI Unveils UK's Disclosure Site For Payments to Health Professionals
Disclosure UK, a new database requiring pharma companies to declare payments made to healthcare professionals, will record any payments made by companies to UK healthcare professionals for attending continuing professional development events, associated travel and hospitality costs, and any payments for work as advisers or consultants to companies.
Korean Authorities Mull New Steps, Probe Over Olmutinib Events
Additional safety measures including a possible sales suspension are to be discussed by a review committee in South Korea on Oct. 4 that will help decide the fate of Hanmi’s EGFR inhibitor olmutinib. Financial authorities in the country are also launching a probe into the timing of the disclosure of several cases of serious adverse events with the drug.
French Trial Tragedy: Experts Recommend Tighter Phase I Rules
The French committee investigating the data behind the Phase I study of Bial's FAAH inhibitor BIA 10-2474, which resulted in the death of one volunteer and the hospitalization of five others in January 2016, has made a number of recommendations for tightening up the regulations on first-in-human studies.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.