Axsome Soars After Depression Trial Success
$1bn+ Peak Revenues Forecast Once Approved
Executive Summary
Axsome is set to file for approval in the second half of 2020.
Axsome Therapeutics Inc. is on track to file its novel depression treatment AXS-05 after scoring a major success in a Phase III trial.
The novel ‘multi-modal’ product met the primary endpoint in its GEMINI Phase III trial of 327 adult patients, rapidly and significantly improving symptoms of major depressive disorder (MDD) compared to placebo.
News of the results sent the New York-headquartered firm’s stock soaring on NASDAQ on 16 December, rising to a peak of $85.30, nearly double the previous day’s share price.
The company now expects to file oral therapy AXS-05 (45 mg dextromethorphan/105 mg bupropion) with the US Food and Drug Administration in the second half of 2020.
Granted a breakthrough designation by the FDA for MDD in March 2019, analysts now tip the therapy to earn around $1bn from its use in this category and in treatment-resistant depression (TRD), for which it is also in late-stage trials.
The study success is all the more stark after rivals Sage Therapeutics saw their MDD candidate fail in its Phase III trial two weeks ago, and will give Axsome an extra advantage in class with few new options reaching the market.
AXS-05 combines two existing off-patent drugs – dextromehorphan (DXO) – best known as an over-the-counter cough medicine – and bupropion (BUP), GSK’s now-generic depression treatment Wellbutrin. The combination aims to create pharmacokinetic synergy because BUP inhibits the otherwise rapid metabolism of DXO, thereby helping to achieve potentially therapeutic plasma levels of the latter.
The drug is a ‘multi-modal’ therapy, active across no fewer than five different existing drug modalities: NMDA receptor antagonist; sigma-1R agonist, norepinephrine reuptake inhibitor; serotonin reuptake inhibitor; dopamine reuptake inhibitor; nicotinic ACh receptor antagonist.
GEMINI Study
Axsome has argued that its therapy offers a more rapid onset of action as well as efficacy compared with existing agents, plus convenience thanks to its oral formulation. Now chief executive Herriot Tabuteau is confident that the new data back up these claims.
AXS-05 met the primary endpoint in the GEMINI study by demonstrating a highly statistically significant reduction in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score compared with placebo at week six, with mean reductions from baseline of 16.6 points for AXS-05 and 11.9 points for placebo.
The trial also showed that rates of remission from depression (defined as MADRS ≤10) were significantly greater for AXS-05 compared to placebo at week two and at every time point thereafter, being achieved by 39.5% of AXS-05 patients compared to 17.3% of placebo patients at week six.
The therapy also showed superiority to placebo in rapid onset of action, and in numerous quality of life measures and questionnaires.
The most commonly reported adverse events in the AXS-05 arm were dizziness, nausea, headache, diarrhea, somnolence, and dry mouth, but these were all within an expected range.
AXS-5 is also in Phase III trials for treatment resistant depression, which will pit it against a new entrant to that market, Johnson & Johnson’s Spravato (esketamine), approved in the US in March 2019. (Also see "EU Approval Delay for Janssen’s Spravato?" - Scrip, 17 Dec, 2019.)
The GEMINI study adds to the successful Phase II trial, ASCEND, in which AXS-05 was compared directly with bupropion alone.
The company does not have any data on the durability of the drug’s effectiveness beyond six weeks, but is running an open-label safety extension trial to collect longer-term data.
Analysts at SVB Leerlink have now increased their forecast for AXS-05 sales, predicting sales in MDD and TRD to be $750m to $1bn by 2030.
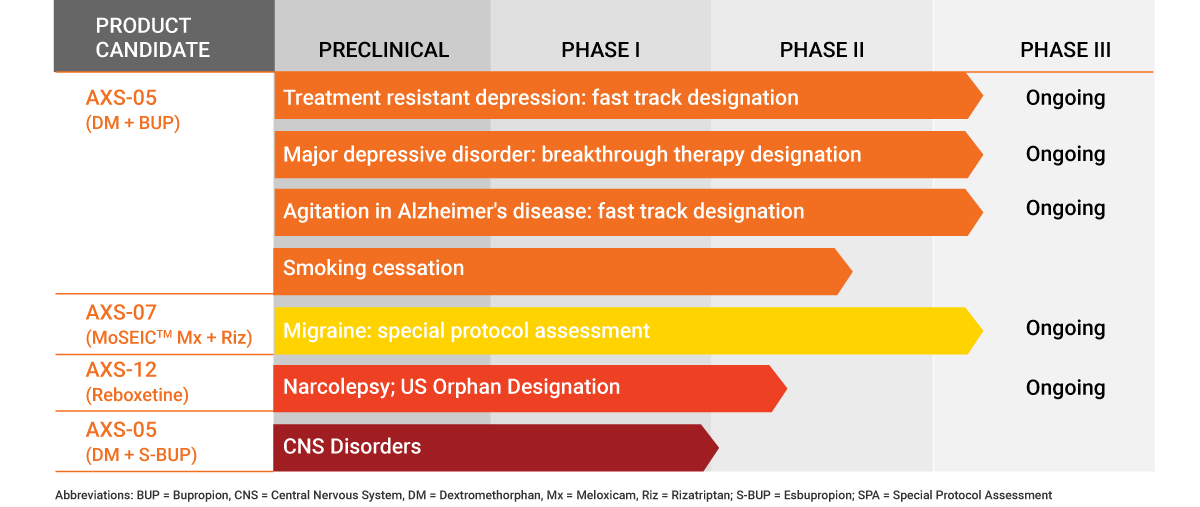
Axsome Therapeutics' Pipeline
That is not all for AXS-05, which is being studied for two more indications: smoking cessation (Phase II) and in Alzheimer’s disease agitation (Phase III) for which it has been granted a fast track designation by the FDA.
SVB Leerlink also upgraded its expectations for another of the company’s pipeline candidates, AXS-12, which is being developed to treat narcolepsy, and which recently posted positive Phase II results.
Key readouts for the next 12 months include a Phase III migrane readout (MOMENTUM) for AXS-07, expected before 2019 year end, and Phase III (STRIDE-1) of AXS-05 in late 1Q20.
Talking to analysts after the data readout, Herriot Tabuteau and chief commercial officer David Marek confirmed that the company planned to go it alone in marketing AXS-05 in the US, but said it would seek a commercial partner for other markets.