Nektar/Bristol Show Promise With Double IO Combo, But Numbers Are Small
Executive Summary
Updated data from PIVOT-02 at the ASCO GU meeting suggest a path for accelerated approval of the NKTR-214/Opdivo combination as first-line treatment of patients with PD-L1-negative urothelial cancers.
It's still early days yet and the numbers are very small, but the double immuno-oncology combination of Nektar Therapeutics' NKTR-214 in combination with Bristol-Myers Squibb Co.'s PD-1 inhibitor Opdivo for advanced urothelial cancer has potential to treat the sizeable population of patients with low PD-L1 expression, based on updated results from the Phase I/II PIVOT-02 study.
Bristol’s well-established checkpoint inhibitor is being paired with a new immuno-oncology (IO) agent: NKTR-214 is a CD-122 agonist, targeting one of the components of IL-2 receptors found on the surface of T-cells. Nektar's pegylated version of human recombinant IL-2 has the effect of delaying breakdown, allowing more convenient dosing and hopefully a better safety profile.
The multi-cohort PIVOT-02 study is testing NKTR-214 – now called bempegaldesleukin – with Bristol's Opdivo (nivolumab) in a range of cancers – urothelial, melanoma, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC) and triple-negative breast cancer – in a total of about 480 participants. The triplet combination of bempegaldesleukin, Opdivo and Bristol's CTLA-4 inhibitor Yervoy (ipilimumab) will also be evaluated in NSCLC and RCC as part of the trial.
Updated results from PIVOT-02 first-line advanced urothelial cancer cohort were released in a poster presentation on Feb. 15 at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) in San Francisco. Lead investigator Arlene Siefker-Radke, professor of genitourinary oncology at the University of Texas MD Anderson Cancer Center, presented results.
Out of 27 patients, the confirmed best overall response rate (ORR) for the combination of NKTR-214 and Opdivo was 48%, including five patients (19%) who got a complete response (CR). The ORR was the same as what was reported in an abstract released after the market closed on Feb. 11, though the later release included four more patients.
The response rate disappointed the market, as the company had previously reported an ORR of 60% (six of 10 patients) in advanced bladder cancer at the ASCO annual meeting in June 2018. (Also see "A Plea For Patience: Bristol/Nektar's Opdivo/NKTR-214 Combo Responses Deepen Over Time" - Scrip, 3 Jun, 2018.)
At the ASCO GU meeting, safety data were available for 41 patients. The rate of Grade 3 adverse events in these participants was 15% and there were no Grade 4 or Grade 5 events. Adverse events included flu-like symptoms (71%), fatigue (56%) and rash (46%). Four dropped out due to adverse events.
Once IO/IO Bitten, Twice Shy
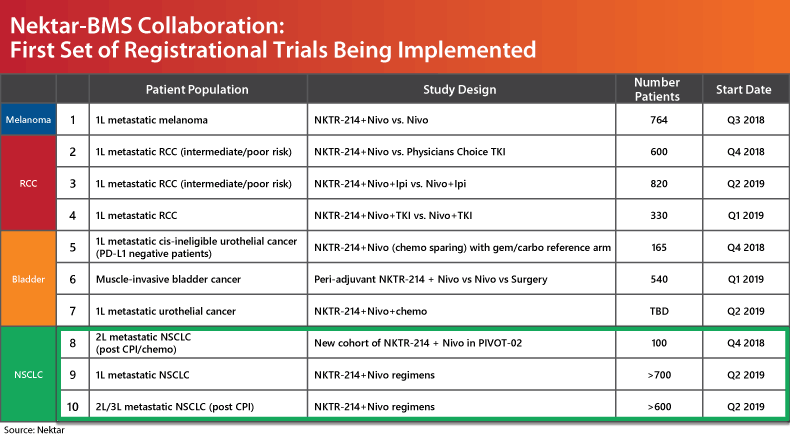
Nektar and Bristol have been closely tied on the development program for the past year; on Valentine's Day 2018 they sealed a deal to develop IL-2 in combination with Bristol's Opdivo and CTLA-4 inhibitor Yervoy in over 20 indications in nine tumor types. (Also see "Bristol Goes In Big On New Valentine Nektar" - Scrip, 14 Feb, 2018.) Per the agreement, Bristol agreed to pay Nektar $1.85bn upfront, including $1bn in cash. (Also see "Nektar/Bristol Deal May Shake Up Immuno-Oncology Landscape" - Scrip, 14 Feb, 2018.) (See chart below for status of registrational trials planned through the partnership.)
Enthusiasm ran high at the time but developments since then have made investors wary about new immuno-oncology combinations. Combinations of IDO inhibitors with PD-1 inhibitors famously flamed out in 2018. (Also see "A Wake For IDO: Bristol Ends Registrational Trials Of High-Priced Flexus Drug" - Scrip, 30 Apr, 2018.) And performance of Bristol's Opdivo/Yervoy combination has paled in comparison to Merck's competing chemo/PD-1 combination. Bristol withdrew an FDA filing for the combination of Opdivo/Yervoy for first-line NSCLC earlier this year. (Also see "Bristol Stuck In Waiting Game As Opdivo TMB Gamble Fails To Pay Off" - Scrip, 24 Jan, 2019.)
Promise of Turning "Cold" Tumors "Hot"
In addition to the 48% overall response rate and 19% CR noted in the latest update, researchers reported a disease control rate of 70%.
In 11 patients known to be PD-L1-negative, the response rate was 45% and in 12 PD-L1-positive patients the ORR was 50%.
Seven PD-L1-positive samples at baseline converted to positive after three weeks and all of the PD-L1-positive patients stayed positive, investigators reported.
The ability to turn tumors from "cold" to "hot" – meaning that they can respond to immunotherapy – is a great hope for IO combination regimens. The same observation was made at the ASCO meeting in June 2018 when the PIVOT-02 data were presented, with excited clinical experts commenting on how it was remarkable to see activity in PD-L1-negative tumors with conversion to PD-L1-positive after treatment.
"This is definitely something that is taking the field by storm – this whole understanding that there are tumors that are cold to the immune system, tumors that are intermediate and tumors that are already warm. And we are seeing differential response patterns based upon the expression of PD-L1 markers," Siefker-Radke said told Scrip in an interview at the meeting.
And as with melanoma, investigators are reporting a deepening of response over time in advanced bladder cancer, she said.
Merck's PD-1 inhibitor Keytruda (pembrolizumab) and Roche's PD-L1 inhibitor Tecentriq (atezolizumab) are approved in the US as monotherapies for first-line bladder cancer, but are contraindicated for those with low PD-L1 expression due to low efficacy in this population. (Also see "US FDA Fine-Tunes Tecentriq, Keytruda First-Line Bladder Cancer Accelerated Approval Indications" - Pink Sheet, 21 Jun, 2018.)
Unmet Need In PD-L1-Negative Patients
Nektar noted that studies done for both of these drugs suggest that about 70% of the advanced urothelial cancer population has low expression of the PD-L1 biomarker.
Based on labeling, for the general population, the ORR with Tecentriq in patients not eligible for cisplatin chemotherapy is 23.5%, with a 6.7% CR. For Keytruda, the ORR in that population is is 29% with a 7% CR. In those who had low PD-L1 expression, the ORR was 21.8% for Tecentriq with a 6.9% CR. For Keytruda, the ORR was 21% with a CR of 3%.
Chemotherapy is a treatment option for PD-L1-negative disease, but many patients have a hard time tolerating it, Siefker-Radke noted.
And while the main unmet need is for patients who have low expression of PD-L1, the combination also looks promising for those who are PD-L1-positive, she said.
"These are small numbers, so we will need a larger sample size to verity this. But when you look at PD-L1-positive disease, I think it's a very exciting treatment there at well, though with a bit more competition. We will need to see durability of responses that are achieved with immunotherapy combinations and compare that to durability with chemotherapy IO combinations," she said.
"As we look at patients who need treatment, to me this is a very attractive option that brings us away from the field of chemotherapeutic poisons and moves us toward treatment that has the ability to stimulate the immune system," Siefker-Radke added.
Watch This Space
At the time of the ASCO release in 2018, the company advised patience with the melanoma response rates as responses deepen over time and the same holds true for the new bladder cancer data.
"There were small numbers reported at ASCO so you would expect variability in patients' disease. The response rate we are seeing overall is in the 40% to 50% range. It's still early, with median follow-up of only 5.1 months," Siefker-Radtke said.
The Phase II PIVOT-10 trial testing the double IO combination in first-line advanced bladder cancer patients who are ineligible for cisplatin chemotherapy and have low expression of the PD-L1 biomarker started recently, and during a Feb. 15 investor call Chief Medical Officer Jonathan Zalevsky affirmed it is potentially registrational. The primary endpoint is ORR and gemcitabine/carboplatin will serve as a reference arm. In a similar population, gemcitabine/carboplatin had a 36% ORR and a 2.5% CR.
A Phase III confirmatory study testing the combination in first-line muscle-invasive bladder cancer will start in this quarter. The primary endpoint for that study is event-free survival. Patients will be stratified by PD-L1 status.
Analysts' Reaction
In a Feb. 11 note about the ASCO-GU abstract for PIVOT-02, Canaccord Genuity analyst Arlinda Lee described the data as promising. Though the data are early, the combination of NKTR-214 with Opdivo "shows efficacy better than published reports" and the conversion of PD-L1-low patients from negative to positive is "intriguing," Lee said.
"With its broad mechanism of expanding and activating T-effector cells regardless of PD-L1 status and across tumor types, we continue to view NKTR-214 as a promising contender in cancer immunotherapy. We view NKTR’s immunology and oncology pipeline as attractive and reiterate our BUY," Lee said.
Lee also noted Nektar's other partnerships. In addition to working with Bristol, Nektar is doing combination R&D with drugs in Pfizer Inc.'s portfolio including the PD-L1 inhibitor Bavencio (avelumab), which is partnered with Merck KGAA, and the investigational PARP inhibitor Talzenna (talazoparib). (Also see "Deals Shaping The Medical Industry, December 2018" - In Vivo, 19 Dec, 2018.)
Nektar is also partnered with Takeda Pharmaceutical Co. Ltd. to develop NKTR-214 with Takeda's dual SYK and FLT-3 inhibitor TAK-659.
Datamonitor analyst Michael Ramirez commented after the Nektar call on Feb. 15 that the update for PIVOT-02 bodes well for NKTR-214 in combination with Opdivo.
A key feature of the combination is its activity in first-line patients that are PD-L1-low, a patient population with high unmet need for which overall response rates with immune checkpoint inhibitor monotherapy is typically in the range of 20%, with complete responses seen in less than 10% of patients, he noted.
The 45% ORR and 18% CR seen in patients with low PD-L1 expression (< 1%) in this update therefore compares favorably to immune checkpoint monotherapy in the first-line setting, he said.
"Another noteworthy result is the 44% ORR observed in cisplatin chemotherapy-ineligible patients, as the typical ORR range for immune checkpoint inhibitor monotherapy in first-line cisplatin-ineligible patients is between 23%-29%," Ramirez said.
And with no Grade 4 or 5 treatment-related adverse events (TRAEs) observed, and Grade 3 TRAEs seen in only 15% of patients, the combination appears well-tolerated, the analyst concluded.
The durability of response in the PIVOT-10 study will be very important in these patients as that was important in PD-1/L1 approvals in this setting, BTIG analyst Robert Hazlett said in a Feb. 19 note. Data are expected in June 2020.
Given that about 70% of first-line patients that are PD-L1-low are not eligible for cisplatin chemotherapy, BTIG estimates the opportunity in this population in the US alone is worth $1.6bn.
Morningstar's Karen Andersen, however, said that the latest data release doesn't change her skeptical outlook on the data. Only 27 of 41 were efficacy evaluable in the study, she noted.
"While cross-trial comparisons rarely paint the whole picture, a 45%-plus ORR would crush previous late-stage results from PD-L1 inhibitors, especially in PD-L1-negative patients. For example, in Merck's Keynote-052 Phase III study, confirmed ORR was 29% in all comers but 21% (47.3%) in PD-L1-negative (positive) patients. If NKTR-214 and Opdivo's efficacy deteriorates in late stage trials, there may still be room for the combination in PD-L1-negative patients, where the bar is lower. However, we would have to see a clear benefit of the combination over checkpoint inhibitors alone.
"We're still unconvinced of how much NKTR-214 is adding in efficacy compared to Opdivo alone," Andersen said.