Roche's Guide To Success In Oncology
Executive Summary
Roche's potential in the growing field of immuno-oncology has been a game of wait and see. But now that Tecentriq has launched, the company believes the components are in place to maintain its leadership position in oncology.
It's good to be the leader, Roche Pharmaceuticals CEO Daniel O'Day emphasized during the firm's annual oncology review for analysts on the ground at the American Society of Clinical Oncology meeting. It was an understandable theme for a company that has long held the top position in the industry's most competitive therapeutic area, but has fallen behind in the burgeoning field of immuno-oncology.
While Bristol-Myers Squibb Co.'s success with its PD-1 inhibitor Opdivo – a roughly $500m lead over Merck & Co. Inc.'s anti-PD-1 Keytruda driven by lung cancer sales, where it nabbed the first approval – certainly reinforces a first-to-market advantage, Roche recently entered the fray with its own PD-L1 inhibitor, Tecentriq – and no one should ever count Roche out when it comes to cancer.
The firm intends to lead from behind, and rather than focusing on the same initial indications as Opdivo (nivolumab) and Keytruda (pembrolizumab), it brought Tecentriq (atezolizumab) to market in a unique tumor type – bladder cancer – and doubled-down on next-generation therapies and building rational combinations.
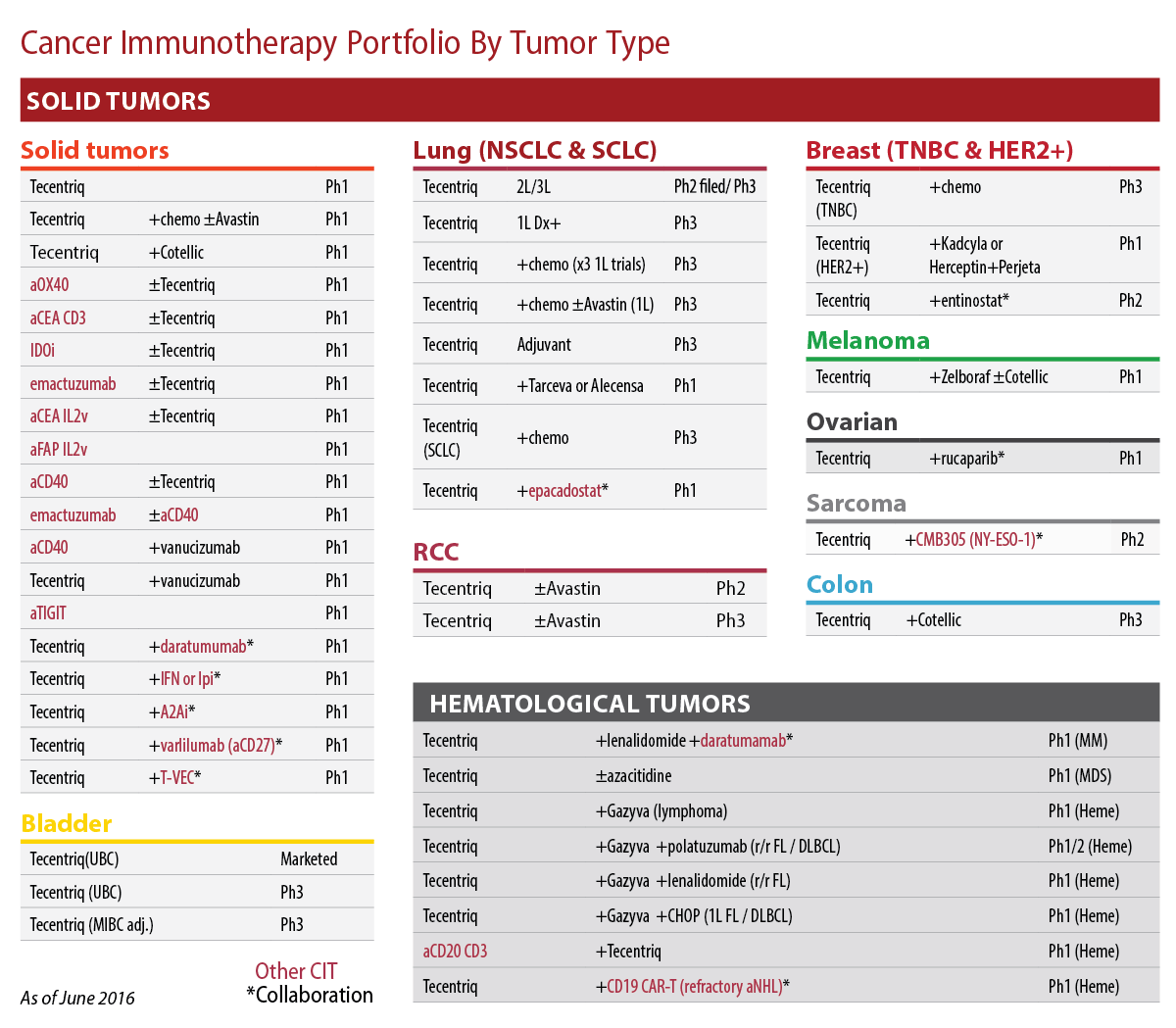
O'Day touted that Roche has launched four new oncologics in the past seven months and has 20 cancer immunotherapy drugs, nine of which are in the clinic. Using Tecentriq as a cornerstone, in the past year Roche has doubled the number of combinations in development. (Also see "For Roche Immuno-Oncology, It’s Steady As She Goes" - Scrip, 24 Jun, 2016.)
The breadth of Roche's portfolio is routinely heralded as a major strength, along with the familiar standards of having good people, following the science and setting the bar high for innovation. "We also put our money where our emphasis is," the CEO added. The Roche group spends close to CHF10bn ($10.4bn) on R&D. More than half of that, close to 60% is dedicated to oncology, he said.
"We're very proud of the fact that we're the leader in breakthrough therapy designations, both in NMEs and in line extensions," and, O’Day added, "we're clearly not done. We've got lots of breakthrough therapy designations that we're in the process of filing or will be filing. But we want to stay in the lead here, because we want that quality level not only to be recognized by us, but be recognized by regulatory authorities, health care authorities, payers around the world."
One thing that also sets Roche apart is its skill at developing franchises and the "dogged pursuit of lifecycle management," as O'Day put it. He said the firm strives to establish a new standard of care and then look for ways to improve upon that.
Using the example of Rituxan/MabThera (rituximab), he explained that it's about "making sure that we explore all convenience benefits for patients" but also "resetting the bar in terms of efficacy or safety or both." The recent early termination of the GALLIUM trial of Gazyva (obinutuzumab), due to the successor anti-CD20 product showing superiority over Rituxan-based regimens in first-line follicular lymphoma, has helped position Gazyva as the new standard of care, and the firm is anticipating the GOYA results, expected later this year, "will be the last piece of the puzzle" in showing Gazyva's superiority over Rituxan.
"But it's not [enough] just to stop there," O'Day said, and rest on the head-to-head evidence that they introduced a better anti-CD20 antibody that is "shown to make a difference to patients." Roche has a comprehensive hematology portfolio with an eye toward "tremendous opportunities still to provide a different patient benefit and patient outcomes above and beyond the good news on GALLIUM," he added. "This philosophy, of course, lends itself also to our HER2 franchise and all the indications in which Avastin has been approved."
Earlier in development, Roche is testing new types of antibodies it has engineered to build these franchises out further. The bispecific antibody vanucizumab, for example, binds to VEGF (like Avastin) and also binds to another angiogenic factor, angiopoietin. (Also see "Engineering Roche’s Next Big Thing: Bispecific Antibodies And Beyond" - Scrip, 30 Jun, 2016.)
Strength In Companion Diagnostics
O'Day also played up a familiar refrain for the diversified Swiss company: having an in-house diagnostics business is a key advantage.
Especially as the biologic understanding grows more complex and big data changes the parameters of drug discovery, "the confluence of these disciplines coming together is allowing this very, very rapid evolution," he said.
Cancer care has gone from a broad approach to more targeted therapies to adding immunotherapies and combining it all together. "That has a lot of implications for us as a company and has implications on how we do research, the complexity of that, identifying targets, finding the best data sources to inform us on the best target. It clearly has an effect on how we do development trials," O'Day said, from the pace to the endpoints to patient recruitment. "And it has a big impact on how we eventually are able to help clinicians in this ever complicated world of helping to identify patients."
Diagnostics are how Roche is going to help itself and help physicians "manage this complexity moving forward."
In addition to the in-house expertise, Roche signed an important deal with Foundation Medicine Inc. last year that has quickly become an integral part of Roche's planning, O'Day said. (Also see "Pharma trial needs drive Roche's $1.2bn Foundation Medicine deal " - Scrip, 12 Jan, 2015.)
"We've got a tremendous opportunity to interrogate the database, which is now 60,000-70,000 highly comprehensive diagnostic patients," he noted, and are moving full-steam-ahead with joint development of a blood-based assay that will connect back in with the Foundation Medicine database as well as an immunotherapy panel "that we think is going to be very instructive when we start to talk about rational combinations."
Overall Roche is moving away from the one-test, one-drug model for testing patients for multiple different mutations at the same time from the same tissue sample, or even a blood sample, which can help with initial diagnosis but also for continuous monitoring and guiding patient care.
Looking Ahead To Real-World Evidence
Roche is squarely on the bandwagon for looking at real-world evidence, both to make better cases for reimbursement but also to facilitate development decisions and to help get faster regulatory approvals.
Roche Ventures was an early investor in Flatiron Health Inc., a cloud-based oncology analytics firm founded by a pair of former Google employees and backed by Google Ventures. In January, Roche led a $190m Series C round and entered a sweeping collaboration; Celgene and Amgen Ventures also participated in the round and Flatiron has expanded collaborations with those firms as well. Flatiron is involved in a number of initiatives working with electronic medical records, analytics for reimbursement, quality measures and value-based care models. It recently began a research collaboration with FDA to look at how real-world evidence captured outside of clinical trials can help assess the safety and efficacy of new cancer drugs, starting with lung cancer immunotherapies.
Flatiron touted at ASCO that its real-world database now has participation from more than 20% of community oncology practices in the US, with aggregated electronic medical records.
"First of all, we think we can recruit trials more effectively through this, particularly when we look at smaller subset mutations," O'Day said. But the system can also clarify disease-stage progression and also could help accelerate line-extension approvals, he suggested. A slide highlighted real-world data submissions for Alecensa (alectinib), Zelboraf (vemurafenib), Avastin and Kadcyla (ado-trastuzumab emtansine).
Perhaps most critically, the real-world evidence can help with payers and health technology assessments, "where HTA authorities, particularly outside the US, are demanding sometimes data that is different than the regulatory authorities."
O'Day stressed that Roche is incorporating different endpoints into its clinical trials, from patient-reported outcomes to immune response measures and early markers like pathological complete response and minimal residual disease.
Leveraging The "Learning Loop"
Roche has highlighted its "smart development" strategies in the past, how it has innovated with the structure of clinical development using adaptive trial designs and responder analysis to find biomarkers and inform, adjust and enrich clinical studies in a continually iterative process. It's also played up its increasing use of data analytics.
The progress made with the immuno-oncology business is evidence of those strategies succeeding (see chart below).
Understanding the complexity involved in the cancer immunity cycle – a topic on which Genentech VP-Cancer Immunotherapy Ira Mellman and Roche Global Cancer Immunotherapy Head Dan Chen have published seminal research – is "something that we really have to understand and understand every last bit of nuance … in order to really optimize the process and move forward accordingly," Mellman told the ASCO briefing.
Roche has developed what it calls a "learning loop" – continuously incorporating research on the immune response to cancer and observing what happens in the clinic "and then informing our clinical colleagues to do a different sort of experiment, who then inform us on what we should really do and evaluate next," Mellman explained. One example of this was Roche's work on combining chemotherapy and immunotherapy, another was combining its MEK inhibitor Cotellic (cobimetinib) and Tecentriq. Both combinations are currently in Phase III trials, and moved through development rapidly.
And in the end, speed matters. "The reality is we have to be doing things faster, we have to be doing things better," O'Day said. "Patients out there are waiting for these new medicines and we've got to find a way to get them to them quicker."
Today's policy environment is tremendously "amenable to patient care," he noted. "What that means for us is things are going very, very fast."
That has implications across the board, from how to do trial design to how to do manufacturing and CMC work. Roche has created its own "internal breakthrough therapy designation status," mimicking the FDA process to identify the compounds with the greatest potential and throwing everything it's got at them. The goal is "seamless development across the organization," O'Day explained.
"Internally, we've been a learning, growing, evolving organization and … with our diversity of approaches across our several research bases, we are now working very much seamlessly to pull this together," he said.
According to Biomedtracker's probability of technical success calculator, Roche has an 88% rate of oncology approvals (versus 86% for oncology peers) and a 50% rate of success from Phase III (versus 46% for oncology peers).
With Tecentriq off to a promising launch and the pipeline poised to start gushing, it will soon be clear whether Roche can sustain its top position on the oncology leaderboard.
Roche's Cancer Immunotherapy Portfolio
Source: Roche
