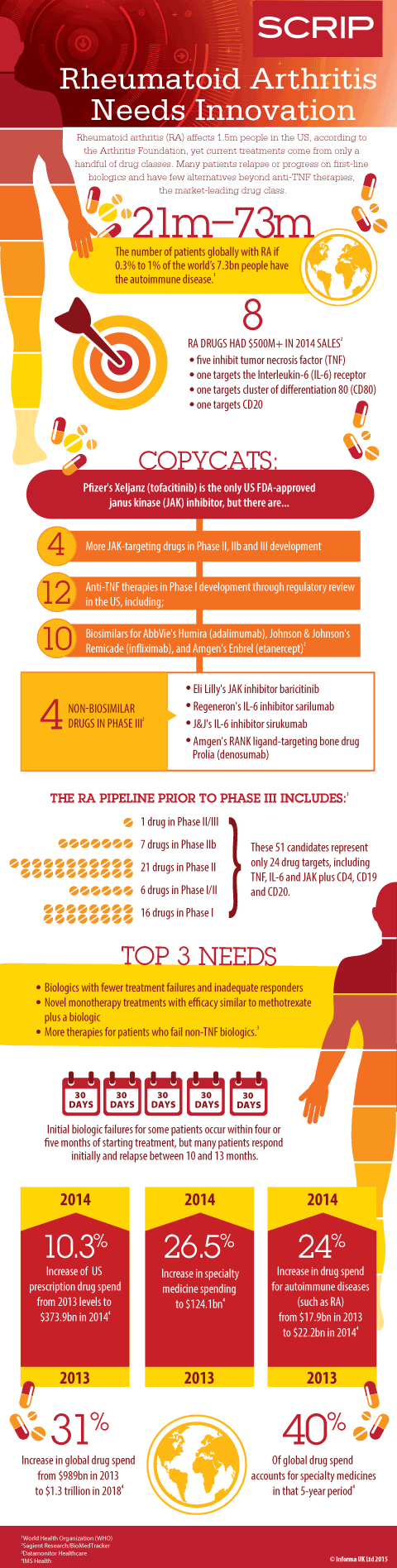
INFOGRAPHIC: RA pipeline has limited new options
This article was originally published in Scrip
If rheumatoid arthritis (RA) patients don't respond to tumor necrosis factor (TNF) inhibitors, they have few options for alternative treatments.
A company called Selventa may have a test to identify patients who won't respond to therapies, but there are few approved options for RA beyond TNF-targeting blockbusters from Amgen, AbbVie and others. The infographic below this article paints a picture of what patients need and what's in the RA development pipeline, according to Datamonitor Healthcare's March 2015 RA market report and Sagient Research's BioMedTracker database.
So what is coming in the next few years for patients identified as TNF inhibitor non-responders by a test from Selventa or someone else?
Interleukin-17 (IL-17) was thought to be a good target, but development of those treatments largely has been discontinued in RA. Novartis ended development of its IL-17 inhibitor Cosentyx (secukinumab) in RA, but the biologic was approved in the US to treat psoriasis and the company is pursuing FDA approval in psoriatic arthritis and ankylosing spondylitis. Eli Lilly & Co is pursuing a similar path for its IL-17 inhibitor ixekizumab.
As for new targets in RA, AstraZeneca is expected to begin a Phase III clinical program before the end of 2015 for mavrilimumab, which inhibits granulocyte-macrophage colony stimulating factor receptor (GM-CSFR). Sagient gives the biologic a 26% likelihood of FDA approval, which is 6% above average for RA drugs in Phase IIb.
GlaxoSmithKline and MorphoSys also have a GM-CSF inhibitor known as MOR103, but it is in Phase I/II for RA and Phase I for multiple sclerosis.
As far as treatment alternatives for people who won't respond to or can't take anti-TNF therapies, if biologics targeting Interleukin receptors prove successful in Phase III clinical trials and win regulatory approvals, "then there will be more choice for rheumatologists, and only then would a test likely have an impact on prescribing habits," Datamonitor Healthcare analyst Jacoba Procter said.
Datamonitor expects the IL-6 receptor antagonist sarilumab from Regeneron Pharmaceuticals and Sanofi, which will be submitted for approvals later this year, as well as the Phase III IL-6 antagonist sirukumab from Johnson & Johnson and GlaxoSmithKline to intensify competition only among non-anti-TNF therapies. So far, the consulting firm noted that the IL-6-targeting biologics have not shown a clear treatment advantage over TNF inhibitors or Roche's marketed IL-6 antagonist Actemra.
"The prospects of these clinical candidates directly competing with existing therapies are low as this would require the pipeline agents to demonstrate greater efficacy, safety or cost-effectiveness compared to their established competitors," Datamonitor said in its recent RA market report, which expects biosimilars for Actemra and the Bristol-Myers Squibb CD80-targeting Orencia in 2023 and 2021 to have a bigger impact on sales for those therapies than sarilumab and sirukumab.
Biosimilar impact
Ms Procter said biosimilar uptake is expected to be slow in RA, because of prescribers' long experience with anti-TNF and other biologic therapies, but the lower cost copycats for biologic medicines eventually will steal some market share. Biosimilars or not, payers may not be terribly sensitive to the cost of RA therapies, because they are used to paying for those medicines.
RA is not a market like hepatitis C, Ms Procter noted, which suddenly had new therapies that cost $84,000 for a 12-week course when Gilead Sciences introduced Sovaldi (sofosbuvir) in late 2013.
Epirus Biopharmaceuticals CEO Amit Munshi, whose company is developing biosimilars globally, said prescriptions for biosimilars outside of the US have been strong, because many payers are cost-conscious government health care programs. He noted that 70% of prescriptions for filgrastim (Amgen's neutropenia treatment Neupogen) in the EU are filled with biosimilars.
It's important to note that biosimilars were approved in the EU and Asia before the FDA approved the first biosimilar for the US market, Sandoz's Zarxio (filgrastim-sndz), which was approved in March and may launch in September.
Mr Munshi told Scrip that biosimilar uptake in the US may be slower, but "as the US expands Medicare, Medicaid and [Obamacare health insurance] exchanges, one might expect to see the US evolve in a way that's not too dissimilar to Europe over time." So far, payers in the US are more amenable to biosimilars than physicians, because doctors are wary until they see how the products work in patients compared with the innovator therapies, he said.
Epirus's lead development program is BOW015, a biosimilar for J&J's Remicade (infliximab). The product is approved in India and the company is pursuing additional approvals in Asia and Europe.
Infographic credit: Gayle Rembold Furbert and Janet Haniak
