Five NCEs Approved In China In 2011: A Closer Look At SFDA’s Progress Report
This article was originally published in PharmAsia News
Executive Summary
China’s SFDA recently released its third annual drug registration report. The agency approved 718 drugs and APIs in 2011, including five NCEs.
SHANGHAI – After a silent 2010 without a single new chemical entity approved in China, the State FDA approved a record five in 2011.
SFDA approved 718 chemical drugs, active pharmaceutical ingredients, biologic products and traditional Chinese medicines last year, according to the agency’s annual drug registration report released Sept. 29 (See: SFDA Drug Registration Report – Chinese language).
The overall approval numbers declined nearly one-third from 1,000 in 2010, mainly due to a decrease in generic drug approvals from 649 cases in 2010 to 436 cases last year. Imported drug approvals declined 35% from 114 in 2010 to 74 in 2011 (Also see "China Drug Approvals Up 26%, Mostly Due To Generics" - Scrip, 19 Oct, 2011.).
However, SFDA approved five Class I drugs last year, while in 2009 and 2010 the agency approved only Youpeng (antofloxacin), the first novel fluoroquinolone anti-bacterial drug developed by Chinese scientists at Shanghai Institute of Materia Medica, and marketed and manufactured by Anhui Global Pharmaceutical, Inc. A Class I drug is defined by SFDA as a drug that has not been approved previously in another country.
One NCE that has received a lot of attention is Beta Pharma Inc.’s non-small cell lung cancer treatment Conmana (icotinib), an EGFR inhibitor that is a reformulation of AstraZeneca PLC’s Iressa (gefitinib) and Roche’s Tarceva (erlotinib). Conmana gained fast-track approval and achieved RMB 100 million in sales in the first seven months following market launch (Also see "Meet China’s New Innovators: Beta Pharma Redefines Innovation With “Me-Too, Me-Better” Strategy" - Scrip, 3 Aug, 2012.). Eli Lilly & Co.’s Lilly Asian Ventures invested an undisclosed amount in a Series A financing for Beta Pharma to help fund completion of clinical research and registration for icotinib.
SFDA also approved Jiangsu Hengrui Medicine Co. Ltd.’s COX-2 inhibitor imrecoxib and its API, a non-steroidal anti-inflammatory drug (NSAID) used to treat rheumatoid arthritis. It is the first Class I drug from Hengrui to be approved by SFDA. The company began developing the compound in 1998.
Also for rheumatoid arthritis, Simcere Pharmaceutical Group’s Iremod (iguratimod) got a nod from the SFDA to launch in China last year (Also see "China's Simcere Beats Out Eisai In Iguratimod Approval For Rheumatoid Arthritis" - Scrip, 31 Aug, 2011.).
The fourth Class I drug is a TNF modulator F647 (pirfenidone) to treat patients with idiopathic pulmonary fibrosis (IPF) developed in China by Shanghai Genomics Inc. The Chinese company is controlled by Tokyo-listed GNI Ltd., a clinical-stage biopharmaceutical company, with its major drug discovery and clinical research teams based in Shanghai (Also see "China Could Be Third Entry For Idiopathic Pulmonary Fibrosis Drug Pirfenidone" - Scrip, 8 Jan, 2010.).
The last Class I drug is claimed by Otsuka Holdings Co. Ltd.’s China operation Zhejiang Otsuka Co. Ltd. The company’s Samsca (tolvaptan) for treatment of dilutional hyponatremia received SFDA clinical trial authorization in 2006, and the NDA was submitted in 2008. U.S. FDA approved the drug in 2009.
|
Drug Category |
2009 |
2010 |
2011 |
||||||||||
|
Locally Manufactured Drugs |
Chemical |
NCEs & APIs* |
2 |
0 |
10 |
||||||||
|
New Drugs for China |
173 |
103 |
93 |
||||||||||
|
Generics |
356 |
640 |
431 |
||||||||||
|
New Formulations |
17 |
51 |
35 |
||||||||||
|
Biologic (including vaccines) |
38 |
12 |
25 |
||||||||||
|
TCMs |
92 |
80 |
50 |
||||||||||
|
Imported Drugs |
Chemical |
100 |
95 |
68 |
|||||||||
|
Biologics (including vaccines) |
13 |
18 |
4 |
||||||||||
|
TCMs |
1 |
1 |
2 |
||||||||||
|
Total |
792 |
1000 |
718 |
||||||||||
Source: SFDA
* In China, APIs are regulated as drugs and included in SFDA statistics
More NCEs On The Way
SFDA also reported an increase in clinical trial authorizations for NCEs in 2011. The agency approved a record 39 NCEs to enter clinical trials in China, up from 32 in 2010 and a three-fold increase from 13 NCEs in 2009.
SFDA also received 3,620 drug/API registration applications, up nearly 20% from 3,066 applications in 2010. Applications from foreign sponsors rose to 707 from 648 in 2010 and 614 in 2009.
On the other hand, applications for global multi-center clinical trials dropped to 110 from 158 applications in 2010 and 132 in 2009, but it is still much higher than prior to 2009, when roughly 40 applications were filed with SFDA annually, reflecting increasing focus on bringing innovative products to China by multinational drug companies.
Global Multi-Center Clinical Trial Approvals (2005-2009)
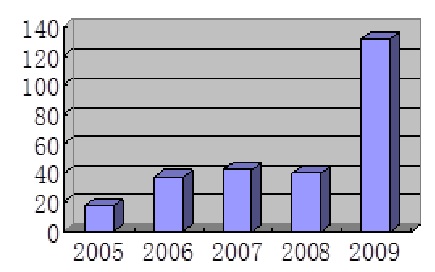
Source: SFDA
Applications For Global Multi-Center Clinical Trials (2005-2009)
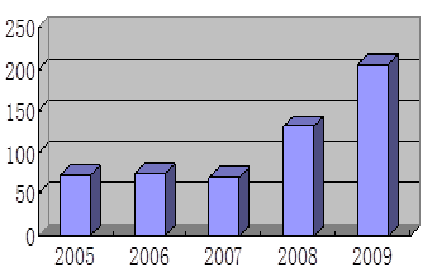
Source: SFDA
MNCs See Diabetes Approvals
While domestic companies won approvals for two rheumatoid arthritis drugs and a cancer drug to compete with Iressa, MNCs also gained two important diabetes drug approvals in China during 2011 with Novo Nordisk AS’s once-daily glucagon-like peptide-1 agonist Victoza (liraglutide) and Bristol-Myers Squibb Co.’s oral dipeptidyl peptidase-4 inhibitor Onglyza (saxagliptin).
Onglyza’s China approval was just two years later than U.S. FDA’s nod in July 2009, and Victoza was even faster, lagging just one year from U.S. FDA approval in January 2010 (Also see "Victoza Approved In China: Novo Nordisk Gears Up To Conquer China's Diabetes Market" - Scrip, 23 Mar, 2011.).
Novartis AG’s DPP-4 inhibitor Galvus (vildagliptin), which was approved by the European Medicines Agency in 2008, but has not been approved by U.S. FDA, was launched in China earlier this year (Also see "After Januvia And Onglyza, Novartis Launches Galvus In China; Lucentis Receives SFDA Nod" - Scrip, 19 Jan, 2012.).
In addition, BMS’s Sprycel (dasatinib) was approved for treatment of Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML).
Johnson & Johnson alsogot approval for its once-monthly schizophrenia drug Invega Sustenna (paliperidone).
Finally, Lilly’s osteoporosis drug Forteo (teriparatide) was also approved by SFDA in 2011. Lilly plans to launch more than a dozen new products and line extensions in China by the end of 2015 (Also see "After Running Lilly From China, CEO Says Local Commitment, Innovation Remain Priorities" - Scrip, 5 Apr, 2011.).
SFDA Continues To Evolve
In China, clinical trials must be conducted in GCP-certified sites. As of now, 356 sites are GCP-certified in China for 2,438 therapies, according to the SFDA report. For preclinical research, China has issued GLP certification to 46 research institutions, four of which have also been certified GLP-compliant by the Organization for Economic Cooperation and Development, and two have passed U.S. FDA’s GLP inspections.
SFDA began to conduct GMP inspections in 2011 on foreign drug manufacturers that export drugs to China. In the past year, the agency has visited seven countries including the U.S., France, Italy, India, Hungary, South Korea and Japan, said the report.
In August, SFDA released a draft regulation on overseas inspections providing details on key documents that will be required for imported drugs (Also see "China Looks To Step Up Overseas GMP Inspections With New Draft Guidance" - Scrip, 30 Aug, 2012.).
One other noteworthy item in the report: SFDA said it will shorten its CTA review timeline to five to eight months from nine to 10, following a restructuring of its drug review body, the Center for Drug Evaluation (Also see "China's Center For Drug Evaluation Remakes Itself To Enter New Era Of Transparency, Speed Up Approvals" - Scrip, 11 Mar, 2011.).
Drug Registration Process In China
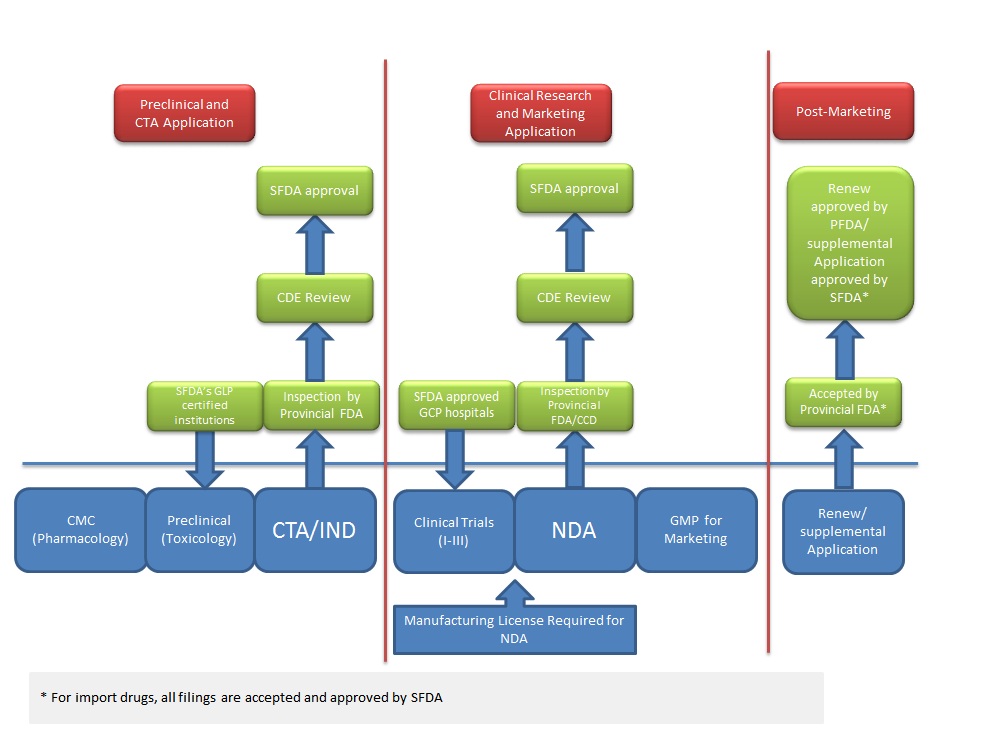
Source: SFDA