Mind over matter - the missing link: Alzheimer's disease and unmet need
This article was originally published in Scrip
Alzheimer's disease is probably the biggest area of unmet need in the field of neurology. Despite extensive research, there is still no disease-modifying therapy available. We look at clinical trial activity in this area.
Like many other therapeutic categories in the CNS area, new products in development for the treatment of Alzheimer's disease include a number of improved formulations of existing products. The best illustration of this is probably Eisai's Aricept (donepezil): a jelly formulation was approved and launched in Japan last year. The product is designed for patients who have difficulty swallowing tablets or fine granule formulations, and is sufficiently soft that it can be broken up with the tongue and swallowed without water. Eisai is following up this product with a sustained release tablet formulation, which was filed for approval in the US last September (the NDA was accepted for review in February 2010), as well as a transdermal patch formulation intended for once- or twice-weekly dosing.
Donepezil itself is now a relatively old product, and two European companies, Applied Pharma Research of Switzerland and Labtec of Germany, are collaborating on the development of a RapidFilm formulation of donepezil hydrochloride in oral film strips. RapidFilm is a proprietary Labtec technology that is claimed to combine the application ease of a liquid with the stability and dosing accuracy of a tablet. The product being developed with Applied Pharma Research is intended for use by patients with swallowing difficulties, and cannot be spat out once taken.
Another example of the reformulation approach is Namenda ER, a once-daily, extended-release formulation of memantine being developed by Forest Laboratories. Memantine is an orally-available amantadine derivative originally developed by Merz as Akatinol/Akura (it is also marketed by Lundbeck as Ebixa) for the treatment of Alzheimer's disease, neuropathic pain and AIDS-related dementia. A US filing for Namenda ER is in preparation.
Formulations aside, there is however a great deal of activity in terms of developing new products for Alzheimer's, and a broad spectrum of mechanisms of action are being investigated. Just to take late-stage products as an example, Pfizer is developing bapineuzumab, a humanised monoclonal antibody designed to neutralise β-amyloid peptides; Lilly is developing semagacestat, a small-molecule γ-secretase inhibitor, as well as solanezumab, a humanised monoclonal antibody that binds to β-amyloid peptide; Medivation is developing latrepirdine, a preparation of dimebolin hydrochloride that targets the ionotropic glutamate receptor; and Martek Biosciences is investigating the potential of docosahexaenoic acid.
While the rationale for targeting β-amyloid is obvious, the basis for targeting γ-secretase is that this enzyme is one of those responsible for the formation of β-amyloid, and its inhibition results in the formation of shorter amyloid chains that are believed to be less toxic to cells. The role of the ionotropic glutamate receptor is a little less clear, although it is thought to be involved in several types of neurodegeneration. The precise target of Martek Biosciences' docosahexaenoic acid is unknown. Altogether, as Figure 1 shows, there are more than 25 different targets under clinical investigation in the search for new Alzheimer's treatments.
| Figure 1: Principal pharmacological targets of Alzheimer's drugs in clinical development |
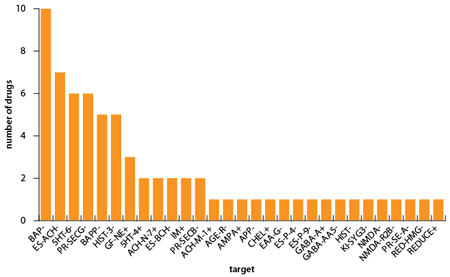 |
| Source: Citeline Drug Intelligence Service; accessed 21 April 2010 |
Unsurprisingly, perhaps, the greatest area of interest is in β-amyloid and its precursor protein, but 5HT receptors, particularly 5HT6 receptors, histamine H3 receptors and γ-secretase are all attracting considerable attention. As well as Pfizer's bapineuzumab and Lilly's solanezumab, products in development that target β-amyloid include Pfizer's humanised IgG2?A monoclonal antibody ponezumab, which is designed to be immunologically inert and to eliminate complement fixation compared with IgG1 monoclonals; Morphosys's gantenerumab and AC Immune's MABT-5012A, which are both licensed to Roche; and Toyama's T-817MA, which is being studied in combination with donepezil.
All the products in clinical trials that target 5HT6 receptors are antagonists, consistent with the observation that blockade of central 5HT6 receptors increases glutamatergic and cholinergic neurotransmission. Potentiation of cholinergic transmission is, of course, precisely what is achieved by cholinesterase inhibitors like Aricept, so the basis for using 5HT6 antagonists is immediately apparent. 5HT6 antagonists in clinical trials include:
- Pfizer's SAM-531, which is in a Phase II trial in 72 patients with mild-to-moderate Alzheimer's to assess its safety, tolerability, pharmacokinetics and pharmacodynamics, as well as in patients with schizophrenia;
- GlaxoSmithKline's GSK-742457, which is also currently in Phase II trials scheduled to be completed within the next couple of months;
- Avineuro's AVN-101, which is in Phase II trials for Alzheimer's and which appears also to have potential in anxiety and depression;
- Avineuro's AVN-322, currently in Phase I;
- Suven Life Sciences' SUVN-502, a highly-selective and orally-active 5HT6 antagonist under development for the relief of Alzheimer's symptoms and expected to enter Phase II later this year; and
- a hitherto unidentified 5HT6 antagonist being investigated by Pfizer.
The histamine H3 receptor was discovered about 20 years ago, and is thought to be involved in the regulation of the release of various neurotransmitters. As such, it is an attractive drug target for a number of indications, not least Alzheimer's disease. Blockade of central H3 receptors enhances the release of neurotransmitters including acetylcholine, a property reminiscent of the 5HT6 antagonists and, in addition, H3 antagonists have been found to enhance cognition in experimental studies.
This particular avenue of research is being explored by several major pharmaceutical companies, including Abbott, GlaxoSmithKline and Sanofi-Aventis, as well as some smaller ones. Abbott's ABT-288 is in a Phase II trial in Russia and the Ukraine, while GSK's GSK-239512 is expected to complete a German Phase II study next year. Sanofi-Aventis's SAR-110894, meanwhile, is still undergoing Phase I studies.
Besides Lilly, companies exploring γ-secretase inhibitors include Pfizer, Humanetics and Eisai. The most advanced is Humanetics's NIC5-15, a natural product that is the result of a research collaboration with the Mount Sinai School of Medicine in New York. It is in a US double-blind, dose-escalation, placebo-controlled pilot Phase IIa trial in patients with mean age 70 years with mild-to-moderate Alzheimer's disease. Pfizer's begacestat, meanwhile, arose from a joint project between Wyeth and ArQule. The product is currently in Phase I, as is Eisai's E-2012.
Figure 2 shows the companies that are carrying out clinical trials in Alzheimer's disease. While current market leader Eisai is fairly active, as already discussed, the Japanese company focuses on new formulations of older products as much as on new molecular entities, and it has been eclipsed somewhat by other major companies that have recognised the potential of the Alzheimer's market. The obvious examples are Pfizer and Merck & Co, neither of which currently has a major product of its own in this area (although Pfizer co-promotes Aricept with Eisai in the US).
| Figure 2: Companies with Alzheimer's drugs in clinical development |
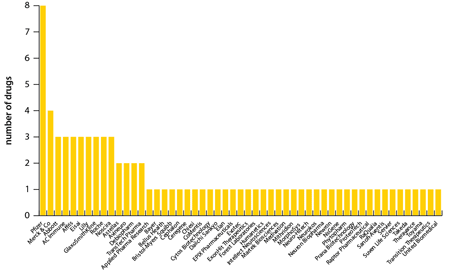 |
| Click on the image to zoom Source: Citeline Drug Intelligence Service; accessed 21 April 2010 |
Apart from the four products mentioned above, Pfizer's other interests in this area are: ACC-001, a β-amyloid CRM immunoconjugate in Phase II acquired along with Wyeth; PF-4447943, a phosphodiesterase IX inhibitor also in Phase II; and PF-5212377, another former Wyeth product in Phase I. For its part, Merck is carrying out Phase II trials with MK-0249, which is in Phase II, and three products in Phase I, including V950, a vaccine against an undisclosed target.
