ASCO 2023 – A Role For Immuno-Oncology In Ovarian Cancer At Last?
Executive Summary
AstraZeneca’s Duo-O trial suggests that excluding Brca-positive patients could be the key to Imfinzi’s apparent success in ovarian cancer.
AstraZeneca PLC appears to have teased out a positive result for Imfinzi in ovarian cancer – a setting that so far has proved intractable for anti-PD-(L)1 drugs. The data, toplined positive in April, concern the Duo-O study of an Avastin/Imfinzi/Lynparza triplet, and have been presented 3 June as an American Society of Clinical Oncology (ASCO) late-breaker.
The result is notable for having apparently succeeded where other PD-(L)1/Parp inhibitor combinations have failed, and one reason for this might be Astra’s exclusion of Brca-positive patients, who would normally be expected to do well on Avastin/Lynparza alone. Still, questions will remain about Imfinzi's contribution, the breadth of the effect, and the robustness of a progression-free survival endpoint.
Debate continues about the validity of PFS in ovarian cancer, and there have been cases where a clinical benefit on PFS has been followed by a clearly negative result in terms of overall survival. This has, for instance, seen use of GSK’s Parp inhibitor, Zejula, narrowed in ovarian cancer maintenance.
Complexities
Duo-O had a complex three-arm design, and included two settings. Active cohorts comprised chemo/Avastin/Imfinzi first line followed by Avastin/Imfinzi with or without Lynparza in the maintenance setting; this was compared against a control cohort of chemo/Avastin followed by Avastin maintenance.
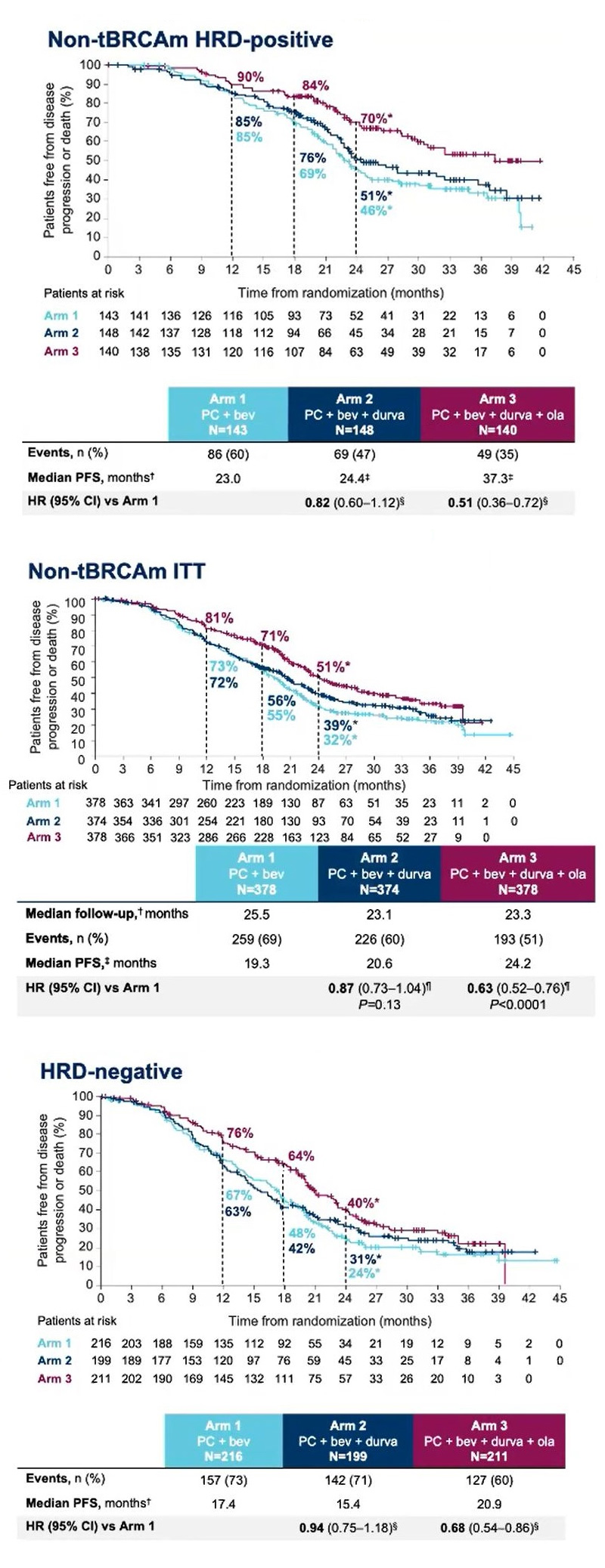
A further crucial twist is that the trial enrolled Brca-negative patients only, and its PFS endpoint was split between two co-primaries: an effect in all-comers, and in Brca-negatives who were nevertheless positive for some other type of HRD mutation.
The good news is that the Avastin/Imfinzi/Lynparza maintenance triplet met both co-primaries, with p<0.0001. The bad that Avastin/Imfinzi had no advantage over control at all.
The survival curves reveal another nuance. It might have been assumed that HRD-positive patients are driving the all-comers benefit, but in fact in HRD-negatives, some 60% of the Duo-O Brca-negative population, the triplet also beat control.
Source: Dr Carol Aghajanian & Asco.
That Parp inhibition should work in patients with no HRD mutation seems counterintuitive. Presenting Duo-O at a pre-ASCO press briefing, Memorial Sloan Kettering’s Dr Carol Aghajanian suggested that these patients might not be HRD-negative at all, since the tests on which their HRD status was based “are imperfect”.
Understanding the extent to which HRD-positives are actually responsible for any benefit is key to estimating the possible breadth of any label here. And of course nothing is yet known about OS, a secondary Duo-O endpoint that is still immature.
A separate question is how much benefit Imfinzi brings; notably only the addition of Lynparza to the maintenance stage yielded a positive result. “If we had to do it again of course we would have a fourth arm that had Avastin and Lynparza maintenance,” said Aghajanian.
Whether Imfinzi is active here goes to the heart of why Duo-O’s positive hit is unexpected. Immunotherapy has tended not to work in ovarian cancer: witness the failures of Roche Holding AG’s Imagyn-050, Merck KGaA/Pfizer Inc.’s Javelin Ovarian 100 and Merck & Co., Inc.’s Keynote-100 trials.
Next to test the theory will be Merck & Co’s Keylynk-001 trial, which tests Keytruda followed by Lynparza in the maintenance phase, and like Duo-O enrolled only Brca-negative patients. The Athena-Combo trial of Opdivo and Rubraca was to have read out in the first quarter, but its sponsor, Clovis, has gone out of business.
|
PD-(L)1 + Parp inhibition in ovarian cancer |
|||||
|
Trial |
Setting |
Active treatment |
Comparator |
Note |
Brca restriction? |
|
Roche |
|
||||
|
≥2L |
Tecentriq + Rubraca |
Uncontrolled |
Ended 2020 after Covid-related protocol amendment, no data reported |
None in ovarian cancer cohort |
|
|
Merck KGaA/Pfizer |
|
||||
|
1L & maintenance |
Chemo + Bavencio, then Bavencio + Talzenna |
Chemo +/- Avastin, then Talzenna or Avastin |
None evident |
||
|
Merck & Co and/or Astrazeneca |
|
||||
|
1L & maintenance |
Chemo + Avastin + Imfinzi, then Avastin + Imfinzi +/- Lynparza |
Chemo + Avastin, then Avastin |
Maintenance triplet positive for PFS in HRD+ves, all-comers & HRD-ves |
Must be Brca-ve (including other HRD+ves) |
|
|
1L & maintenance |
Chemo + Keytruda, then Lynparza |
Chemo +/- Keytruda, then placebo |
PFS in PD-L1+ves & all-comers are co-primaries**; ends Oct 2023 |
Must be Brca-ve |
|
|
Bristol Myers Squibb/Pharma& (ex Clovis) |
|
||||
|
1L maintenance |
Opdivo + Rubraca |
Opdivo or Rubraca or placebo |
PFS primary, data were due Q1 2023*** |
None evident |
|
|
Note: *not phase 3; **earlier PFS & OS were co-primaries; ***forecast made by Clovis, which later entered bankruptcy and sold Rubraca to Pharma& for $70m. Source: company statements & clinicaltrials.gov. |
|||||
– Jacob Plieth ([email protected])
This article originally appeared in Evaluate Vantage. Evaluate Vantage and Scrip are part of the same parent company, Norstella.