ASCO 2023 – Elevation Follows In Astellas’s Slipstream
Executive Summary
There is reason for the enthusiasm behind Elevation’s anti-Claudin18.2 antibody-drug conjugate, but no such good news for Zai Lab’s zolbetuximab challenger.
Armed with two pivotal study hits Astellas Pharma, Inc.’s zolbetuximab is headed for the regulators later this year, and now comes the next wave of projects that also hit Claudin18.2. Investor focus recently fell on one of these, Elevation Oncology, Inc.’s SYSA1801, and the 3 June data presentation at the American Society of Clinical Oncology (ASCO) annual meeting appeared to back up some of the enthusiasm.
There was less good news for Zai Lab Ltd.’s ZL-1211, which despite being supposedly enhanced for activity showed no responses in the poster presented 3 June. The question for Elevation, a micro-cap valued at just $86m, is whether SYSA1801’s antibody-drug conjugate modality can maintain a manageable safety profile.
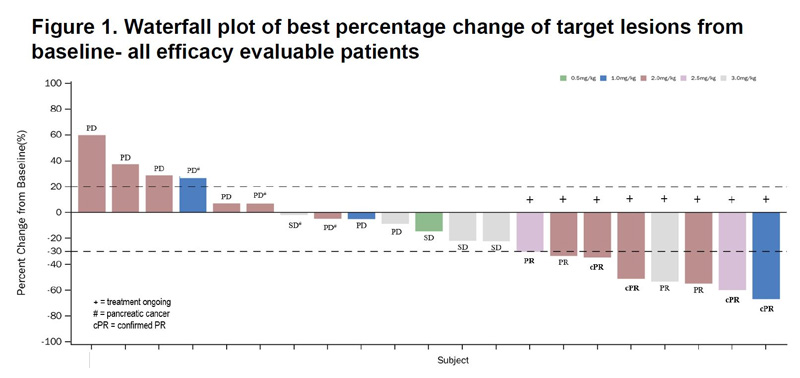
So far the signs are that nausea and vomiting are the most common treatment-related adverse events, and a potential concern is that in two of seven subjects given the highest SYSA1801 dose of 3mg/kg – 33 were enrolled in total – this tox was dose limiting.
The ASCO presentation contained the same 5 November 2022 data cutoff as detailed in the abstract that sent Elevation up 67% a week ago. Why Elevation brought data that were nearly seven months old to ASCO is another question.
Still, given the company’s undemanding valuation perhaps some enthusiasm is warranted. There were 21 evaluable Claudin18.2-positive patients at data cutoff, 17 of whom had advanced gastric cancer, and among these there were eight partial responses, four of which were independently confirmed.
Source: Elevation & Asco.
The 47% ORR here looks impressive given that this is a monotherapy study. In comparison zolbetuximab’s pivotal Spotlight trial, in Claudin18.2-positive gastric cancer, yielded ORR of 61%, though this was a chemo combo study. Curiously chemo alone gave 62%; that puzzling finding only slightly detracted from the trial’s demonstrated survival benefit.
Zai Lab
While SYSA1801 is an ADC, Zai’s ZL-1211 is, like zolbetuximab, a naked MAb. However, unlike the Astellas project it has been souped up with site mutations introduced into its Fc region, with the aim of enhancing antibody-dependent cellular cytotoxicity.
The ASCO poster detailed 10 evaluable Claudin18.2-positive patients, but the best result seen was stable disease with 25% tumour reduction in a gastric cancer patients. Serious nausea and vomiting occurred in one of 19 safety-evaluable subjects, but in none given high ZL-1211 doses, so perhaps Zai has scope to dose higher still.
– Jacob Plieth ([email protected])
This article originally appeared in Evaluate Vantage. Evaluate Vantage and Scrip are part of the same parent company, Norstella.