Pricing In ‘Exceptional’ Times: ICER Aims To Shape US Policy For Novel COVID Drugs, Vaccines
Executive Summary
US Institute for Clinical and Economic Review lays out options ranging from the status quo (unrestricted pricing) to compulsory licensing and advanced market commitments in a new white paper.
The time is now for stakeholders to find consensus on how to price novel vaccines and treatments for COVID-19, the Institute for Clinical and Economic Review emphasizes in a white paper released 2 July.
“Ultimately, policymakers must determine who will own the treatments that society needs, and how much they will cost,” the paper says. “These questions are not new, but in the exceptional circumstances of the coronavirus pandemic their answers will guide decisions in the coming months that will have enormous short and long-term consequences for the United States and the rest of the world.”
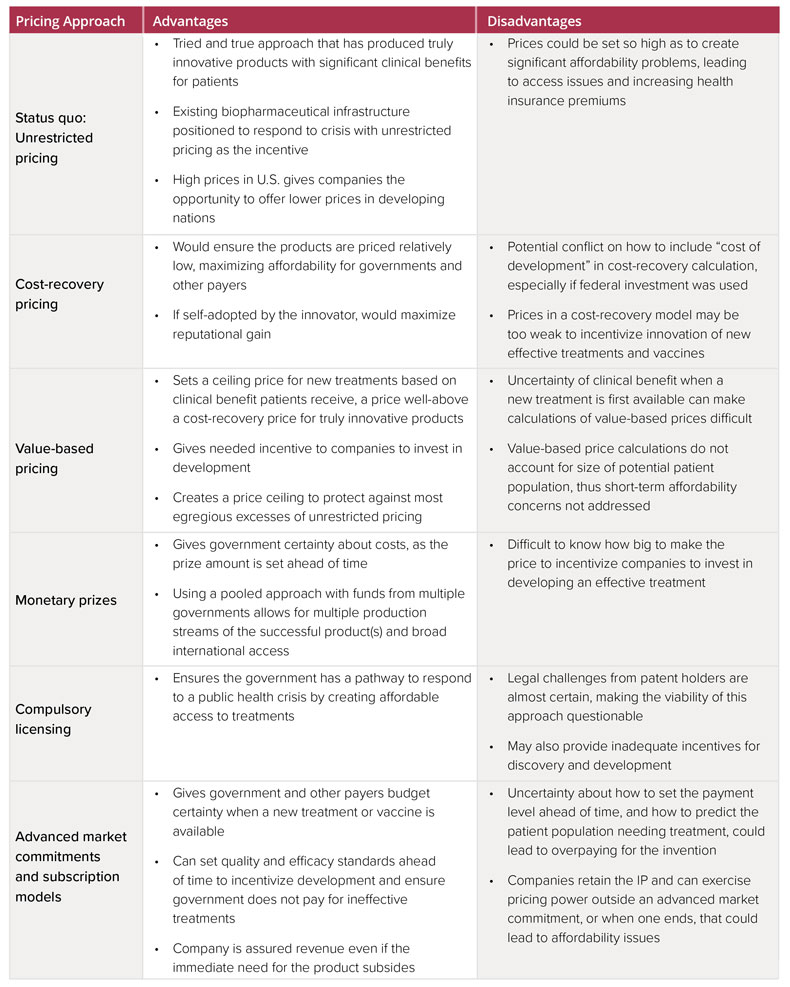
The white paper lists six approaches to pricing and explores the advantages and disadvantages of each (see chart below). In its discussion of the various models, ICER acknowledges the importance of providing financial incentives for development of the first wave and subsequent novel treatments and vaccines for COVID-19. But the group also emphasizes the need to head off “egregious excesses of unrestricted pricing" that could overwhelm government and payer budgets and lead to restrictions on access.
As a “complement” to the white paper, ICER will convene a series of three public webinars in the coming weeks allowing stakeholders to debate the approaches. Participants already scheduled include a congressional staffer, a financial analyst, drug pricing policy experts, patient advocates and former US Health and Human Services Department assistant secretary for preparedness and response Nicole Lurie.
ICER isn’t specifically backing any of the proposals in the white paper, a spokesman for the group said. It expects “the diverse group of panelists at our events will weigh in on the pros and cons of each of these options, as well as their feasibility — including the aspects that would require Congressional or Executive action to accomplish.” By facilitating the discussion, “we’re hoping to give policymakers the information they need to begin implementing one or several of these approaches,” he added.
The initiative is aimed specifically at pricing for novel drugs and vaccines, ICER explained. “Drugs already in clinical practice may be found to be effective for COVID-19 but the considerations around appropriate pricing for these drugs, particularly generic drugs, are different,” the group pointed out.
“The US should continue to explore alternatives to a drug pricing system that leaves the decision about the prices for drugs that may mean the difference between life and death for millions of people in the hands of a single company, without competition, and whose only constraint on pricing is their own moral conscience enforced by public pressure.”
ICER earlier released proposals regarding appropriate pricing for Gilead’s remdesivir in COVID-19 and has expressed support for the prices recently announced by the company. (Also see "Gilead’s Remdesivir Pricing Sets ‘Promising’ Precedent For Future COVID-19 Drugs" - Pink Sheet, 30 Jun, 2020.) But this latest initiative is rooted in the concern that a consensus-based policy with broader application is needed.
“The US should continue to explore alternatives to a drug pricing system that leaves the decision about the prices for drugs that may mean the difference between life and death for millions of people in the hands of a single company, without competition, and whose only constraint on pricing is their own moral conscience enforced by public pressure,” ICER said in response to Gilead’s pricing announcement.
Three of the approaches in the white paper involve private companies developing vaccines and treatments and retaining patent rights: the status quo, cost recovery pricing and value-based pricing.
The status quo of unrestricted pricing is a “tried and true approach that has produce truly innovative products with significant clinical benefits,” the paper notes. However, “prices could be set so high as to create significant affordability problems, leading to access issues and increasing health insurance premiums,” ICER pointed out.
The next two options would involve governments and/or private insurers setting a ceiling price. Cost recovery pricing, which would only recoup manufacturing and development costs, could ensure low prices but “may be too weak to incentivize innovation,” particularly for research into preventive treatments that would come after the first wave of therapies is introduced.
Value-based pricing, which is the technique used by ICER, involves a cost-effectiveness analysis scaled to the health and economic benefits of a new treatment. However, the approach could be complicated by uncertainties over clinical benefit when a COVID-19 treatment is first available and would not account for the size of potential patient populations or consider payer cost burdens, ICER noted.
Ceding Patent Control To The Government?
Under the next two options, the government would control the patents to novel treatments or vaccines, an approach that is unlikely to find traction among biopharma stakeholders. Under one of the two options, the government would establish a monetary prize to incentivize discovery of a vaccine, with the winning company awarded the prize in return for the intellectual property. The government would then contract separately with entities to manufacturer and distribute the product at cost.
The other options is compulsory licensing, which would involve the government paying royalties to the innovator in return for control of the intellectual property, an arrangement that would allow the government to permit others to make, use, sell or import the treatment without the patent-holder’s consent.
On mechanism for implementing compulsory licensing could be for the National Institutes of Health to exercise “march-in rights” under the 1980 Bayh-Dole Act, the paper suggests. The provision would allow the government to seize or transfer control of a patent to ensure an invention is available to the public on “reasonable terms” or when action is needed to alleviate “health or safety needs.”
However, NIH director Francis Collins has said that drug pricing concerns would not justify such action. (Also see "NIH Reluctant To “March In”: Collins Suggests Authority Not Intended To Address Pricing Concerns" - Pink Sheet, 20 Apr, 2016.) And legal challenges to compulsory licensing from patent holders would be “almost certain, making the viability of this approach questionable,” ICER observed. Compulsory licensing may also provide “inadequate incentives for discovery and development,” the paper acknowledges.
Advanced Market Commitment
Finally, an advanced market commitment could subsidize research and development costs through a commitment by a funder (a government or group of donors) to a future purchase price, if the development is successful, ICER proposes.
The funder could guarantee payment for a successful product, thereby eliminating the uncertainty a developer faces when investing in a development program without certainty of a return. Innovators would retain intellectual property control under the model. And the government would determine in advance the total price it would pay for as many doses as are needed to control COVID-19.
A subscription pricing model, such as was recently introduced in Louisiana for hepatitis C treatment, is a related approach that could be employed, ICER noted. (Also see "Louisiana Subscription Contract For Generic Epclusa About Access, Not Discounts " - Pink Sheet, 7 Aug, 2019.)
However, uncertainties about how to set payment and how to predict the patient population needing treatment could lead to overpayment in the models, ICER pointed out. In addition, because companies would retain patent rights, they could “exercise pricing power outside an advanced market commitment, or when one ends, that could lead to affordability issues.”