Cyclerion Looking To Partner Praliciguat Despite Phase II Fail In Diabetic Nephropathy
Executive Summary
The Ironwood spinout thinks its sGC stimulator demonstrated benefit in reducing urine albumin levels in DN patients; notes data improve markedly if one outlier trial site is excluded.
Cyclerion Therapeutics Inc. is still hoping to find a partner for its soluble guanylate cyclase (sGC) stimulator praliciguat for diabetic nephropathy despite reporting a pair of unsuccessful Phase II studies on 30 October; the disappointing news was paired with the announcement of plans to reduce its staff by 25%-30%, causing an 80% drop in its share price.
The company finished the trading day at $2.75 per share, but CEO Peter Hecht told Scrip that he still expects potential partners will be persuaded of praliciguat’s potential value in DN when they see the full dataset. “We missed the primary endpoint, [but] this is an exploratory study and the data both on the primary and other measures of kidney function – particularly if you look at it in the various forms of analysis we did – give us strong confidence that we’re seeing the attributes of a very important medicine,” he said.
The Cambridge, MA-based firm spun out from Ironwood Pharmaceuticals Inc. in April with $175m in funding to focus on the parent company’s sGC pipeline, which also includes Phase II olinciguat for sickle cell disease and Phase I IW-6463, the first sGC compound able to cross the blood-brain barrier. (Also see "Cyclerion Readies For Readouts In Sickle Cell, Other Rare, Serious Diseases This Year" - Scrip, 9 Apr, 2019.)
Cyclerion reported that praliciguat also failed a Phase II proof-of-concept study in heart failure with primary ejection fraction (HFpEF), but said it saw no reason to go any further with that development effort.
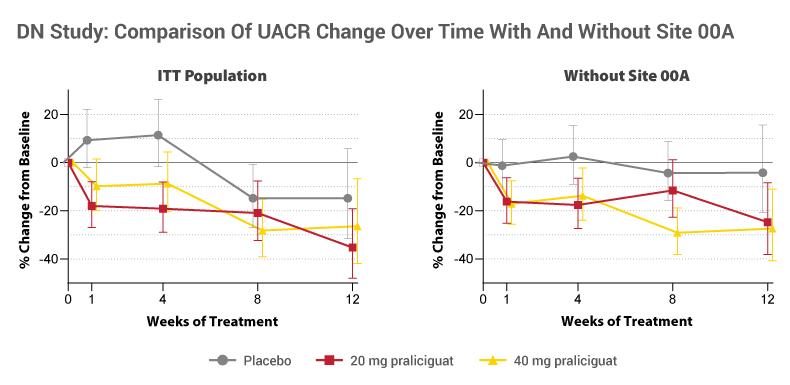
The Phase II study in DN tested 20mg and 40mg doses of daily, oral praliciguat against placebo in 156 patients with a primary endpoint of reduction from baseline in urine albumin creatinine ratio (UACR). For the full study population, Cyclerion saw a positive trend for the drug compared to placebo on this endpoint, but it missed statistical significance within the full intent-to-treat population.
Cyclerion pooled results from the 20mg and 40mg cohorts and found an average of 27.8% reduction in UACR for weeks eight and 12 of treatment, compared to a 14.8% reduction for the placebo arm. The placebo-adjusted average of eight- and 12-week results was a 15.3% improvement (p=0.1736). At week 12, the trial yielded a 30.9% reduction in UACR for study drug and 14.8% for placebo. This meant an 18.9% placebo-adjusted improvement on the primary endpoint (p=0.1956).
The drug also showed improvements in multiple secondary endpoints measuring vascular and metabolic outcomes, including blood pressure, cholesterol and HbA1c levels, the company noted. Chief medical officer Chris Wright wouldn’t clarify whether statistical significance was met for the secondary endpoints. “We’re just talking about general directions of improvement in those endpoints,” he said, noting the full dataset will be reported at a future medical meeting.
Cyclerion noted that praliciguat was well tolerated in the study with a safety profile suitable for continued development. Discontinuation rates due to adverse events were 4% in the placebo arm, 8% in the 20mg arm and 12% in the 40mg arm. Serious adverse events, all of which were deemed unrelated to the study drug, occurred in 2% of the placebo and 20mg cohorts and 8% of the 40mg cohort.
Strategy For Praliciguat Still Centers On Partnering
Hecht said Cyclerion’s intent from its inception has been to take praliciguat through Phase II proof-of-concept in DN and HFpEF and then partner the drug with a larger, cardiovascular-focused company in either or both indications. The goal remains to do so for DN, he said, adding that potential partners were the “primary audience” for these Phase II results.
“We thought we could provide the most value and reduce the most risk by bringing it to this point,” the exec explained. “Not only have we been in conversations with leading cardiometabolic players, but they actually had input and suggestions into the design of the study. There are quite a number of interested parties and we’ll be sharing the datasets with all of them shortly.”
Cyclerion’s pitch likely will hinge on how potential partners respond to its argument that outlier data from one trial site skewed the overall dataset. At that site, a “significantly greater” percentage of patients receiving either of the two doses of praliciguat had undetectable or very low plasma concentrations of the drug than seen in the overall study population, the company found.
While Cyclerion still does not know exactly why the drug plasma levels were lower at the one site, it conducted a sensitivity analysis in which it re-estimated the primary endpoint in a subgroup (n=133) excluding the patients from that one site. That analysis showed an average 23.5% reduction in UACR at eight and 12 weeks for the 20mg and 40mg arms, compared to a 4.2% reduction for the placebo group. The placebo-adjusted improvement was 20.1% (p=0.0303). In the 12-week data, there was a 26.1% reduction in UACR with study drug and 4.1% decrease with placebo. Here, the placebo-adjusted improvement was 22.9% (p=0.0672).
Hecht and Wright admitted they can’t explain why excluding the patients from the outlier site led not to a finding of greater study drug effect but of a reduced placebo effect. Hecht noted that responses were seen in both the treatment and control arms at the outlier site. In addition, he argued that graphs illustrating the response curve for the full ITT population and the subgroup analysis show a fairly consistent picture of rapid and sustained lowering of UACR with both doses of the study drug.
 Cyclerion argues that praliciguat's treatment effect curve is clear
Source: Cyclerion Therapeutics
Cyclerion argues that praliciguat's treatment effect curve is clear
Source: Cyclerion Therapeutics
“There’s something about the cohort of patients at that site that led us to analyze the data with and without that group because there’s a lot about that site that’s implausible,” Hecht said. “We’re going to dig in further, but because this is a Phase II study, not a registrational study, what you’re really trying to do is learn about your drug. The two datasets are directionally, with or without this site, comparable. The signal is clearer, there’s less variance, there’s statistical power when you take out the aberrant site. But it’s not like you have to take that group out to see any [treatment effect] pattern.”
For the 196-patient Phase II CAPACITY study of praliciguat in HFpEF, the drug did not meet statistical significance on a primary endpoint of improved exercise capacity at 12 weeks from baseline compared to placebo. This endpoint was measured on the basis of cardiopulmonary exercise testing.
Cyclerion noted that the drug yielded tolerability and safety data similar to the DN study, and also showed a positive trend for HbA1c lowering in a subgroup of HFpEF patients who also have diabetes. Hecht said these data might be useful in building out a data package for a company working to advance praliciguat toward approval for DN.
Hecht said the staff cut is intended to reduce Cyclerion’s R&D spend rate, which he described as pretty high from the company’s inception in April. The biotech’s focus now turns to the Phase II STRONG-SCD study of olinciguat in sickle cell disease that is expected to report data in 2020 and in determining the indications for Phase I IW-6463, which previously has been categorized generally for central nervous system disorders.
Wright said the focus for ‘6463 is going to be severe neurodegenerative disorders of a metabolic nature that include mitochondrial disease.
Hecht said Cyclerion likely will detail the program more fully in December or January.
