Payer Communications: More Clarity, Opportunity And Responsibility Following New FDA Guidance
Thought Leadership in Association with ICON
Executive Summary
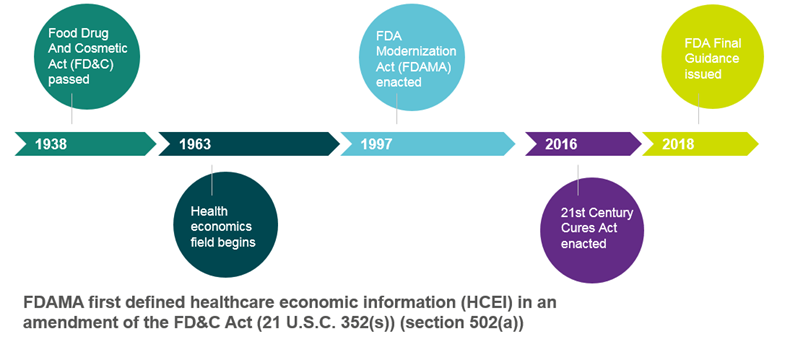
For more than 20 years – from the passage of the US Food and Drug Administration Modernization Act (FDAMA) in 1997 until just this year – the life sciences industry has had little to go on when deciding what health economic information about products could be shared with payers. This was especially true with regard to products or indications that had not yet been granted marketing approval; the boundaries of what was permissible were blurry at best. As a result, most manufacturers of pharmaceuticals and medical devices took a very conservative approach, which in turn forced payers to make formulary and budgetary decisions for their covered populations without all of the information they needed.
Author
By Nathan White, Senior Vice President, Integrated Access and Outcomes Solutions Access, ICON
In June of 2018, the Food and Drug Administration (FDA) released two final guidance documents that eliminate most (but not all) of the uncertainty that manufacturers have faced: “Drug and Device Manufacturer Communications with Payors, Formulary Committees, and Similar Entities – Questions and Answers” and “Medical Product Communications that Are Consistent with the FDA-Required Labeling – Questions and Answers”. Here we review the most significant provisions of these two documents and outline the key takeaways for manufacturers in a recap of a webinar, “Industry Analysis: Impact of FDA’s Recent Guidance on Payer Communications”.
Figure 1. Timeline Of HEOR Communication With Payers
New, Definitive Concepts Related To Communicating With Payers
The guidance on communicating with payers provides far greater clarity in four areas, that despite being quite basic, had been sketchy in the past:
- The Definition of Health Care Economic Information (HCEI)
The FDA defines HCEI as any information that conveys the economic consequences related to the clinical outcomes of treating a disease (or specific aspect of a disease) or of preventing or diagnosing a disease. An HCEI analysis can compare one product with another, with another intervention, or with no intervention. Such information can be presented in an evidence dossier, publication reprint, budget impact model, modelling software, slide presentation or payer brochure, amongst others.
- The Definition Of Payers And Similar Entities
The guidance relates specifically to payers, formulary committees and similar entities. This, by the agency’s definition, includes drug information centres, technology assessment panels and pharmacy benefit managers. The FDA recognises that they represent a “sophisticated audience”… able to “closely scrutinise information about medical products … including an evaluation of the limitations and reliability of that information”.[1] The guidance does not relate to communication with health care professionals (HCPs; except when they are serving in a capacity as one of the above entities) or consumers.
- The Approved Indication
HCEI on approved products must relate to an approved indication – in other words, to the disease, manifestation or symptoms associated with a disease or condition in a patient population for which the drug is indicated in FDA-approved labelling. (The guidance covering communications consistent with FDA labelling is discussed in greater detail under “Communication Consistent with Labelling” below.
- The Required Evidentiary Support
The evidence presented as HCEI must follow the CARSE principle, meaning that it must be Competent, Reliable, Scientific Evidence. A foundational requirement is that the HCEI must not be considered false or misleading. The study data must be accurately represented, and the study design, methodology and limitations must be clearly disclosed. Anecdotal evidence from customers, magazine and newspaper articles, and sales materials do not qualify as CARSE.
Unfortunately, the agency’s guidance does not delineate clearly between a scientific exchange and promotional activity. In general, it is clear that a scientific exchange is educational in nature, unsolicited and delivered by Medical Affairs. In contrast, promotional activity has a sales bent to it, and is delivered by Marketing and Sales. Subject to greater scrutiny by the FDA, promotional information is overseen by the Office of Prescription Drug Promotion (OPDP). It is important to realise that an audience receiving communication that is deemed promotional is not required to keep that information confidential, so payers could, conceivably share such information.
The Academy of Managed Care Pharmacy (ACMP) is continuing to seek clarity on this subject, stating that it is “looking forward to working with Congress, the FDA, and stakeholders … to provide additional clarity to manufacturers and payers regarding truthful and non-misleading communications pre-FDA approval”.[2]
Pre-approval Information Exchange: A Breakthrough For Manufacturers And Payers Alike
The FDA’s final guidance on HCEI differs from the draft document in that it also creates a “safe harbour” for manufacturers to discuss HCEI with payers on unapproved products or unapproved uses of marketed products in a pre-approval information exchange (PIE). In other words, within the confines of HCEI, manufacturers can now discuss off-label uses and not-yet-approved products with payers.
This provision satisfies the longstanding need of payers and population health policymakers for information about treatments 12 to 18 months in advance of marketing approval to develop their budgets and make coverage decisions. Now, manufacturers can share information on the indication being sought, the anticipated timeline for approval, the expected product pricing, patient utilization research and information on product-related programs/services. This information exchange must be a factual presentation of clinical data and not veer into value messages. Details should include the study design, methodology and full findings, confirmation that the product/use is not yet approved, and a copy of the most recent FDA-required label.
Communications Consistent with Labeling
As noted above, communications of a product’s HCEI must relate to the FDA-approved labelling. The FDA has clarified what constitutes medical information that is Consistent with the FDA-Required Labelling (CFL) by explaining what is not CFL. If the answer to any of the following questions is “yes,” then the communication is considered inconsistent.
Acceptable HCEI Topics
- Duration of treatment
- Practice setting
- Burden of illness
- Dosing
- Persistence
- Patient subgroups
- Length of hospital stay
- Validated surrogate endpoints
- Clinical outcome assessments or other health outcome measure
- Comparisons
- Adherence and compliance
- Does the information cover different conditions of use from the label? These conditions include:
- Indication
- Patient population
- Limitations and directions for handling, preparing and/or using the product
- Recommended dose or use regimen or route of administration
- Does the communication increase the potential for harm, negatively altering the benefit/risk profile of the product?
- Given the representations of suggestions in the communication, can the product still be used safely and effectively in accordance with the FDA-required labelling?
Examples of topics that would not be consistent with the label include those related to treating or diagnosing a disease other than the approved indication; to an unapproved patient population; to the use of a different treatment modality; or to a different route of administration, dose, dosage form, strength or length of regimen.
Acceptable CFL Topics
- Comparison of safety/efficacy with another product in the same indication
- Adverse reactions
- Onset of action
- Long-term safety and efficacy
- Patient subgroups
- Adherence/compliance
- Pain
- Patient perspectives
- Health outcomes studies
- Method of action
- Tolerability with concomitant medication
CFL communications should relate back to the data on the same topic in the FDA-required labelling. For example, if the communication provides postmarketing rates of adverse events, it should also include the rate of adverse events seen in clinical trials and reported in the FDA label.
Advice For Manufacturers
In light of these changes and clarifications, we advise life sciences companies to take advantage of their newly sanctioned capabilities where appropriate. Doing so should entail:
- Familiarising yourself with the guidance documents, including a review of the examples the FDA provides of what is consistent/inconsistent with the FDA-required label. In an informal poll of the attendees at ICON’s webinar on the subject, over half (54%) had not read either the draft or final guidance.
- Examining your Standard Operating Procedures (SOPs) to ensure that they reflect the new guidance. In the same informal webinar poll, more than two thirds (68%) of attendees had not changed their SOPs as a result of the published guidance.
- Evaluating all payer communications against the standard that they must be truthful and not misleading and consistent with the FDA-required label. As such, the product information must be accurately characterised and contextualised. You cannot “cherry pick” what you share; unfavourable or inconsistent findings must be disclosed and findings must not be overstated.
- Providing updates on changing information. If data that have been shared on an unapproved product or use changes, you have an obligation to issue an update. This requires having a tracking system in place to know what information has been released to whom.
Although the June 2018 guidance from the FDA does not address every question that has long vexed manufacturers with respect to sharing health economic data with payers, it is a major leap forward, not just in terms of clarity, but in the opportunities it affords manufacturers. Perhaps the most beneficial aspect is the provision of a safe harbour for pre-approval information exchange with payers. One area deserving of additional exploration is the delineation between scientific exchange and promotional activity. For now, it is safest to assume that HCEI disseminated to payers is promotional and therefore subject to regulation by the Office of Prescription Drug Promotion.
For more information on HCEI communication, please download our webinar at www.iconplc.com/webinar-hcei
Nathan White is Senior Vice President, Integrated Access and Outcomes Solutions Access, at ICON. He has over 16 years of experience providing pharma and biotech clients advice on global, US and European market access and reimbursement challenges. For more information visit www.ICONplc.com/commercialisation.
[1] “Drug and Device Manufacturer Communications With Payers, Formulary Committees, and Similar Entities – Questions and Answers Guidance for Industry and Review Staff”, FDA, June 12, 2018.
[2] Academy of Managed Care Pharmacy, Press Release, June 13, 2018.