J.P. Morgan Notebook Day 2: Walmsley On Diversity, Amgen's Overseas Cash, Growing Opdivo, Mylan On US Generic Pricing, Boston's Pipeline And J&J In Alzheimer's
Executive Summary
Daily round-up of news and notes from the 2018 J.P. Morgan Healthcare Conference in San Francisco: GSK's Walmsley looks to be a leader on diversity, Amgen talks tax reform and deals, Mylan raises the issue of generic drug shortages, Boston builds out its pipeline, and J&J is dealing in Alzheimer's disease.
Emma Walmsley’s Diversity Moment
GlaxoSmithKline PLC CEO Emma Walmsley had her J.P. Morgan Healthcare Conference debut on Jan. 9 in San Francisco in front of a standing room crowd in the Grand Ballroom of the Westin St. Francis. The audience was eager to hear more about her plans to prioritize pharmaceuticals and innovation. But it also was a feel-good moment at a time when the industry is growing increasingly aware of its diversity problem – particularly in the top leadership ranks.
Gender diversity was a prominent topic at the industry’s biggest business meeting of the year, given the societal movement under way.
Walmsley became the first woman CEO of a big pharma when she took the reins at GSK last year, succeeding Andrew Witty. (Also see "From Witty To Walmsley – The Priorities For GSK's New CEO" - Scrip, 4 Apr, 2017.)
The chief executive kept the J.P. Morgan presentation focused on her first two priorities: delivering growth for shareholders as well as discovering and developing innovative medicines. But during the breakout session that followed, she also acknowledged that being the first woman to break into the top ranks of big pharma comes with a responsibility to lead the industry on the issue of diversity.
“These jobs come – and I'm new at it – with enormous privilege and a tremendous responsibility,” she said after being prompted by an investor to address the elephant in the room. “I recognize the responsibility that I have as a leader … I want to represent diversity in that sense.”
But the industry’s diversity problem isn’t just about gender, Walmsley said. “I am just as fast and focused on diversity of representation in terms of the LGBT agenda, in terms of race, in terms of personality.”
“You cannot be a modern employer in an industry that should be future-facing and modernizing arguably much more aggressively than it is … without being very demanding on this topic, both as an individual, as a CEO, but also as a leadership team and a company,” she said.
It's common sense, Walmsley said, because the industry should represent the population it's serving.
Amgen Looks To Do Deals With Overseas Cash
Amgen Inc. Executive Vice President and Chief Financial Officer David Meline said during the Q&A session following the company’s J.P. Morgan Healthcare Conference presentation that at the very least under US tax reform the company will not see its tax rate go up and the rate is expected to be around 18% to 19% in 2018.
However, like many other biopharmaceutical companies presenting at the meeting, Amgen’s biggest benefit from tax reform appears to be the ability to use its cash held overseas – a stockpile of about $39bn. The legislation, CEO Robert Bradway noted during the Q&A, puts Amgen on a level playing field with its competitors headquartered outside of the US.
More J.P. Morgan Coverage
For other news from the first two days of the conference, see these stories:
J.P. Morgan Notebook Day 1: Tax Reform At Last, Allergan's Job Cuts, Teva Turnaround, Biogen's Cash, And Getting FDA-Friendly
Celgene's $1.1bn Impact Buy Is First Of More Deals To Come In 2018 And Beyond
Deal Watch: As J.P. Morgan Waits For New Deals, Sanofi Takes Care Of Old Business
Pharma Justifies Steep Prices, Plans Fancy Footwork In The Era Of Cures
Shire May Split Up – But Punting On Bigger Decisions For Now
Pfizer Exits Early Neuroscience, But M&A Could Allow Later Re-Entry
So what will Amgen do with all of that money? “Our priority first and foremost is to expand our portfolio,” Meline said, noting that the company is "very active" in business development.
Amgen is under pressure to bring new products to market as some of its blockbusters face biosimilar competition. (Also see "Amgen Focuses On Pipeline As Mature Products Face Declining Demand" - Scrip, 26 Oct, 2017.)
But the company probably isn't likely to execute a large M&A deal, because Bradway and Meline reiterated their prior thinking about deal-making. Amgen is focused on transactions that add molecules within its six main therapeutic areas, accelerate the company’s global build out, and improve its data skills.
“We have increasing flexibility for business development,” Meline said. “We have very stable and very strong cash flow – a good balance of equity and debt capacity.”
After allocating appropriate funds to execute deals, he added, “we will consider the most efficient way to deliver cash to investors” through increased dividends or buybacks.
Mylan’s Bresch Brings Up Shortages As US Generic Drug Pressure Persists
Mylan NV CEO Heather Bresch said there are concerns about generic drug shortages as price pressure on certain high-volume, low-cost generic drugs continues to mount in the US.
“It’s probably the starting point for a lot of conversations with our customers,” she said in an interview. “They are concerned about the products that are high volume.”
Generic drug makers make just cents on some of the most high-volume generic drugs that represent the bulk of the drugs used in the US. Generic manufacturers make most of their profits on the first-to-file ANDAs where they have exclusivity for a limited period of time. The US generic drug market has come under increasing pressure in the last two years, partly due to consolidation in the distribution chain and also because FDA is successfully working through a backlog of ANDAs to get more generics onto the market, in part to lower US drug spending.
The result presents a bit of a conundrum in the generic drug industry. The situation is one of the issues that is posing challenges to troubled Teva Pharmaceutical Industries Ltd., which is in the midst of laying off 25% of its workforce. It’s not the only one, however, and some companies – including Mylan – have been better able to navigate the situation through geographic expansion and complex generics. As part of its turnaround plan, Teva has vowed that it will raise prices on some non-profitable US generic drugs – about 10% of SKUs – or discontinue them. (Also see "J.P. Morgan Notebook Day 1: Tax Reform At Last, Allergan's Job Cuts, Teva Turnaround, Biogen's Cash, And Getting FDA-Friendly" - Scrip, 9 Jan, 2018.)
“We saw the story play out with injectables years ago. Prices were ultra-competitive. It forced people out of the market, and then we had shortages,” Bresch said.
But Bresch said Mylan will make its way through the challenges by remaining steady, relying on its diversification strategies, and being patient.
“I do think things have a tendency to swing extreme and then recalibrate,” she said. “I think we will find ourselves there, and Mylan will benefit from having that steady hand."
Opdivo's Billions: There's More Where That Came From
Bristol-Myers Squibb Co. CEO Giovanni Caforio highlighted the strong sales track record of the company's PD-1 inhibitor Opdivo and multiple billion dollar opportunities in various indications during its presentation at the J.P. Morgan meeting on Jan. 9.
First approved by FDA in December 2014, Opdivo (nivolumab) now has $4.9bn in annualized sales, 14 approved indications in the US, 250 global approvals and over 30 registrational trials are ongoing in multiple tumor types.
Sales in non-small cell lung cancer (NSCLC) in the US in recent quarters have been stable despite greater competition from competitors in the PD-1/L1 family. (Also see "PD-1 Earnings Roundup: Buckle Up For A Bumpy Ride" - Scrip, 15 Nov, 2017.) Some results from the first-line CheckMate 227 study in first-line NSCLC are expected in the first half of 2018.
Caforio also pointed out that there are multiple billion dollar opportunities in other indications, including renal cell carcinoma (RCC). Opdivo is approved for second-line metastatic RCC and a filing for use with the CTLA-4 inhibitor Yervoy (ipilimumab) in first-line metastatic RCC is under FDA review, with an April 18 user fee date.
The first-line RCC market is twice as big as second-line RCC, Caforio noted, and the combination has potential to become the standard of care in the intermediate-to-poor risk segment of the first-line disease. CheckMate-214 is the only Phase III study to show a survival benefit for a new agent in this setting, he said.
"I really feel that renal can be an over $1 billion opportunity for Opdivo," Caforio said.
Opdivo is also rapidly becoming the standard of care in second-line hepatocellular carcinoma (HCC) since approval for this indication in the third quarter of 2017, the exec said. Opdivo already has made its mark in terms of market share, which has come at the expense of Bayer AG's Stivarga (regorafenib).
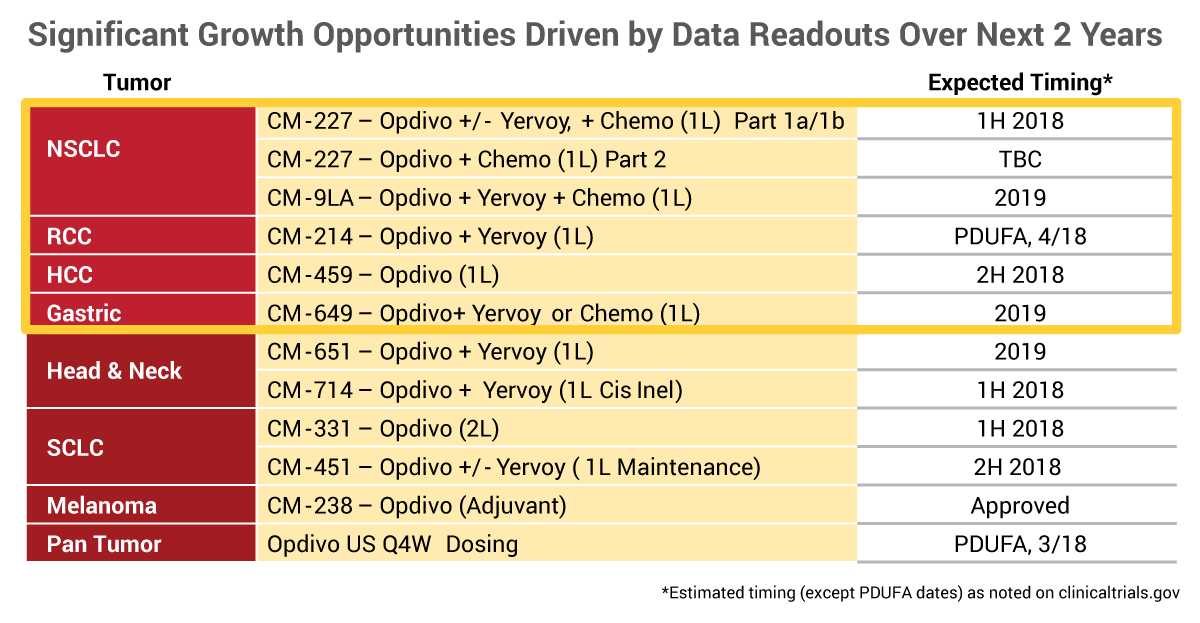
Source: Bristol-Myers Squibb
"This is a small opportunity in second line, but it is an important indicator of the potential role of Opdivo and importantly, of the need for new agents in this disease. In fact, today our share is well above 60% and continuing to grow in second line," Caforio said.
The Phase III CheckMate-459 study of first-line HCC study that will read out in the second half (see graphic).
Gastric cancer represents another billion-dollar opportunity for Opdivo, he said.
Viehbacher-Backed Boston's Pipeline Is Growing, Progressing
Boston Pharmaceuticals Inc. was not a typical start-up when it launched at the end of 2015. It raised $600m from Gurnet Capital – the $2bn investment fund run by former Sanofi CEO Chris Viehbacher – so that it could rapidly acquire and develop compounds de-prioritized by big pharma and other companies or stuck in the valley of death without adequate funding to progress into clinical proof of concept.
The model called for Boston to sell or out-license molecules once they achieved proof of concept – a stage that some of the firm’s acquired assets are just now entering. (Also see "Viehbacher On Boston Pharmaceuticals: A New Model To De-Risk Drugs" - Scrip, 20 Nov, 2015.)
Boston has acquired seven assets – four of which are clinical-stage – and Co-Founder and CEO Robert Armstrong told Scrip in an interview during the J.P. Morgan conference that the company expects to add three to five assets in 2018. The current pipeline includes three autoimmune disease programs, including a lupus drug candidate; two oncology programs, including one in breast cancer; one cardiovascular asset, designed to treat atrial fibrillation; and an anti-infective program.
To date, Boston has reviewed more than 450 molecules. The 22-person company is agnostic when it comes to therapeutic area and drug modality.
“When we created this company, Chris and I recognized that there’s always a disconnect between innovation and the capital that’s available,” Armstrong said. He explained that R&D teams are generating more and more new and innovative molecules, but funding is not keeping up with the need for cash to progress all of those programs.
Both big and small companies struggle to fund all of the programs in their pipelines and frequently put drug candidates on hold to focus on core therapeutic areas, despite a desire to get shelved compounds to patients, as noted by Pierre Fabre Group President and CEO Frederic Duchesne in Boston’s Jan. 4 announcement that it in-licensed global rights to the selective potassium channel blocker F17727. (Also see "Deal Watch: As J.P. Morgan Waits For New Deals, Sanofi Takes Care Of Old Business" - Scrip, 9 Jan, 2018.)
"While our R&D and commercial focus has shifted away from cardiology, we are committed to ensuring that promising compounds are advanced through strategic partnerships to the benefit of patients," Duchesne said.
Smaller biopharma companies reach out to Boston when they are having trouble accessing cash, but also when they have to narrow their focus and look for someone who can develop de-prioritized assets.
“Platform-based companies have always had this problem – they outpace their ability to raise funding and pursue development,” Armstrong said.
Of its seven acquired compounds, three came from pharma and four from biotech. Boston hopes to buy 20 to 25 molecules with its existing capital and plans to spend $25m to $30m each on development of the assets through proof-of-concept data.
And if Boston’s efforts are successful, the economics are attractive for licensors who are patient enough to wait for most of the value from their deals with the company. “The innovator ends up with roughly a third of the value at the back end,” Chief Financial Officer Ian Sanderson said.
J&J Deals In Predicting Alzheimer's Risk
Some of Johnson & Johnson Innovation LLC's latest collaborations are aimed at helping predict risk for Alzheimer's disease early and noninvasively, at a time when the field is still reeling from the latest string of failures.
Johnson & Johnson Innovation is a large initiative of J&J focused on accelerating early research through a range of strategies, including incubators in major biotech hubs, like San Francisco and Boston. (Also see "California Dreaming: J&J Builds Bay Area Ties With Second Startup Home" - Pink Sheet, 6 Mar, 2015.)
On Jan. 8 Axovant Sciences Ltd. announced that its 5-HT6 receptor antagonist intepirdine failed in the Phase II HEADWAY study of dementia with Lewy's bodies. This followed failure in 2017 of the Phase III MINDSET study of intepirdine in Alzheimer's disease. (Also see "Disappointed, Yes, But Roivant's Not Roiled By Axovant's Alzheimer's Failure" - Scrip, 26 Sep, 2017.)
Merck & Co. Inc. terminated the Phase II/III EPOCH study of the BACE inhibitor verubecestat in mild-to-moderate Alzheimer's disease in early 2017 due to lack of efficacy. (Also see "Another Nail In Amyloid Hypothesis Coffin? Merck Ends Pivotal BACE Inhibitor Study" - Scrip, 14 Feb, 2017.)
In an interview at the J.P. Morgan conference, Guy Seabrook, vice president in scientific innovation — neuroscience, said that the intepirdine failure was no surprise – performance hadn't been that hot in Phase II – but Merck's verubecestat had looked very promising in terms of reduction in terms of reducing amyloid levels and failure was a blow.
J&J and other sponsors are not giving up on targeting amyloid, rather they are moving toward disease prevention, before symptoms have even developed. (Also see "Alzheimer's Prevention: The Next Big Idea For Fixing Drug Trial Failures" - Scrip, 27 Sep, 2017.)
Among a dozen new collaborations announced on Jan. 4, just before the J.P. Morgan meeting kicked off, J&J Innovation unveiled a deal with Toronto-based WinterLight Labs, Inc., which has developed artificial intelligence technology that it says can help non-invasively predict dementia and neurodegenerative diseases long before clinical symptoms are apparent.
Early changes in the brain may be detected with positron emission tomography (PET) imaging scans, but this is expensive and Seabrook noted that there are not enough PET centers in the US to allow screening of everybody who is at risk, so there is a need for alternatives, such as WinterLight's artificial intelligence algorithms, which enable testing of the ability to remember faces, names and events.
In another newly announced deal, J&J Innovation said it will be working with the Northern California Institute for Research and Education and the San Francisco Veterans Affairs Medical Center to explore the use of speech recognition technology and neuropsychological assessments for monitoring brain health in elderly people.
"I believe those types of technologies in conjunction with availability of PET will be really quite critical for us to be able identify who is at risk, so if we come up with drugs that are effective at reducing the conversion to Alzheimer's disease we can intervene in an appropriate way," Seabrook said.
J&J will also be working with the University of Pennsylvania's gene therapy program to use Adeno-associated virus vectors for delivering antibodies aimed at Alzheimer's disease.
[Editor's note: The J.P. Morgan Notebook is a shared effort for Scrip and Pink Sheet readers, part of our joint coverage of key industry events.]