A Status Check Of The Alzheimer’s Trial Landscape
Executive Summary
Despite high-profile failures, including the recent disappointing results for Axovant Science’s Phase III MINDSET study, the trial landscape for Alzheimer’s disease remains busy.
Outside the oncology research that dominates pharma R&D, Alzheimer's disease (AD) is one of the more active indications according to Trialtrove. Undeterred by previous failures, the industry continues to mine them for insight, incorporating new strategies and tools in an attempt to address the unmet needs in within the large AD market.
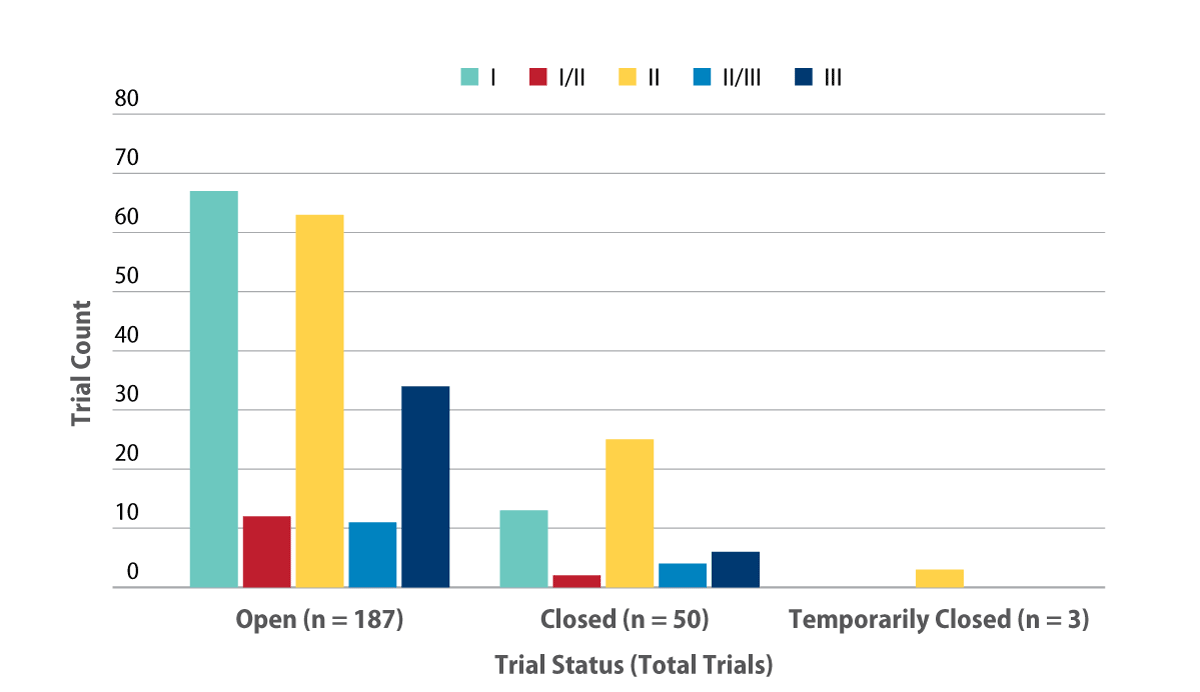
As of October 2017, a total of 240 Phase I to III trials were ongoing for AD – 78% of which were still actively recruiting – exhibiting a healthy level of continuing interest in the area. Most of the remaining trials are closed to enrollment, while three trials are temporarily suspended (see Exhibit 1).
Among the open trials, Phase I activity slightly edges out Phase II with 67 and 63 trials, respectively. Phase III counts are nearly half that and only 34 late-stage trials are actively seeking patients. However, Phase II is the most active development phase for closed trials, which could fuel new opportunities for late-stage research if all goes well for the various drug candidates.
Six Phase III trials are closed to enrollment with varying durations until follow-up for primary endpoints are expected to complete. Most project dates from mid-2018 until 2019, but one trial is expected to continue until July 2022. This long-term trial is Eli Lilly & Co.'s A4 study, taking place in older individuals who have evidence of amyloid plaque build-up to evaluate solanezumab’s effectiveness in preventing AD, continuing development of the antibody that missed its primary endpoints in the Phase III EXPEDITION3 trial.
All three temporarily closed trials are in Phase II, none of which have publicly disclosed a specific reason for their suspensions. The sponsors, and drugs, for these trials are: Boehringer Ingelheim GMBH's BI 425809, Daiichi Sankyo Co. Ltd.'sPlexxicon (pexidartinib), and Fogangren Bio-Pharma and Star Lake Bioscience Co. Inc.'s Zhaoqing Guangdong (memantine hydrochloride).
Exhibit 1. Ongoing Phase I-III Alzheimer’s Trials By Status And Phase
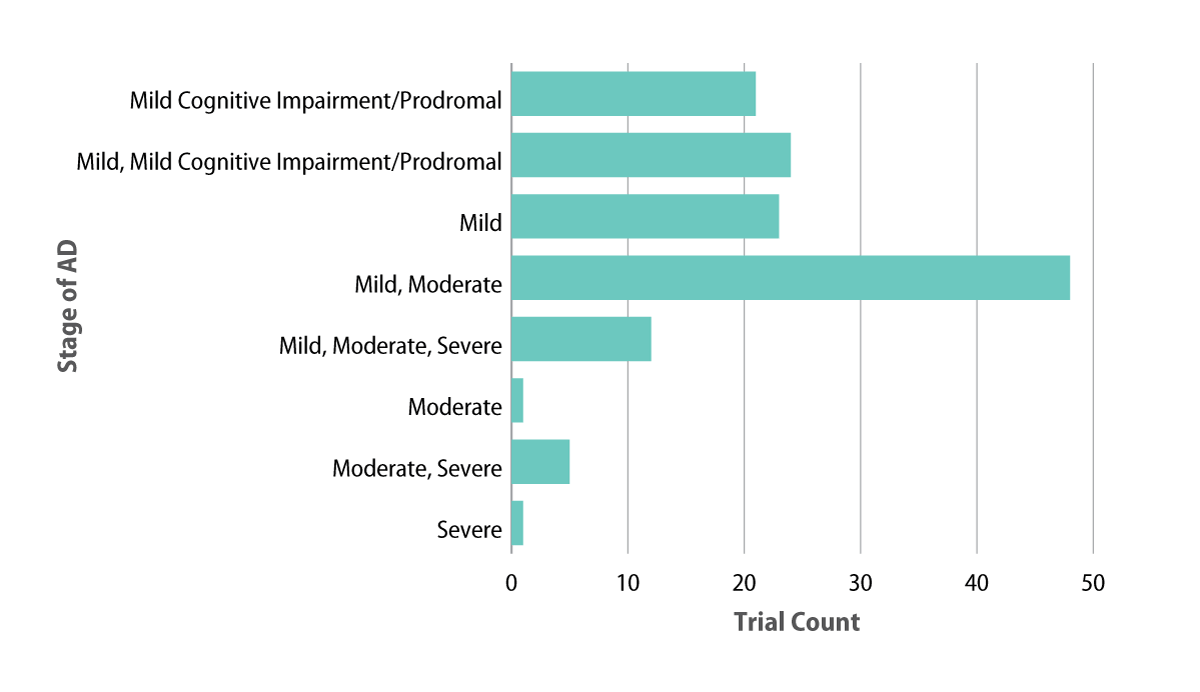
Within AD research, there has been a call to treat patients earlier, or even move towards disease prevention. Patients with mild AD has, and continues to be, the most common patient population included in Phase I to III clinical research. In recent years, this has been coupled with a growing interest in prodromal patients as well as decreasing activity in moderate to severe AD populations. Currently, a total of 54 ongoing trials target patients with mild cognitive impairment and/or prodromal AD, 21 of which only recruits this population. In contrast, seven trials only seek patients with moderate to severe AD, with an additional 12 that include patients with moderate to severe AD, as well as those with mild stages of the disease (see Exhibit 2).
Exhibit 2. Patient Populations In Ongoing Trials
Key Players Driving Current Efforts
Industry companies, excluding those solely focusing on generics, are fueling the majority of ongoing AD clinical research – a total of 144 trials include at least one industry sponsor, or 60%. Academic centers are also prolific and are involved in 100 of the 240 ongoing Phase I to III trials, while government organizations and cooperative groups are only linked to 33 and 18 trials each. Generic developers contribute a modest level of activity with 25 ongoing studies.
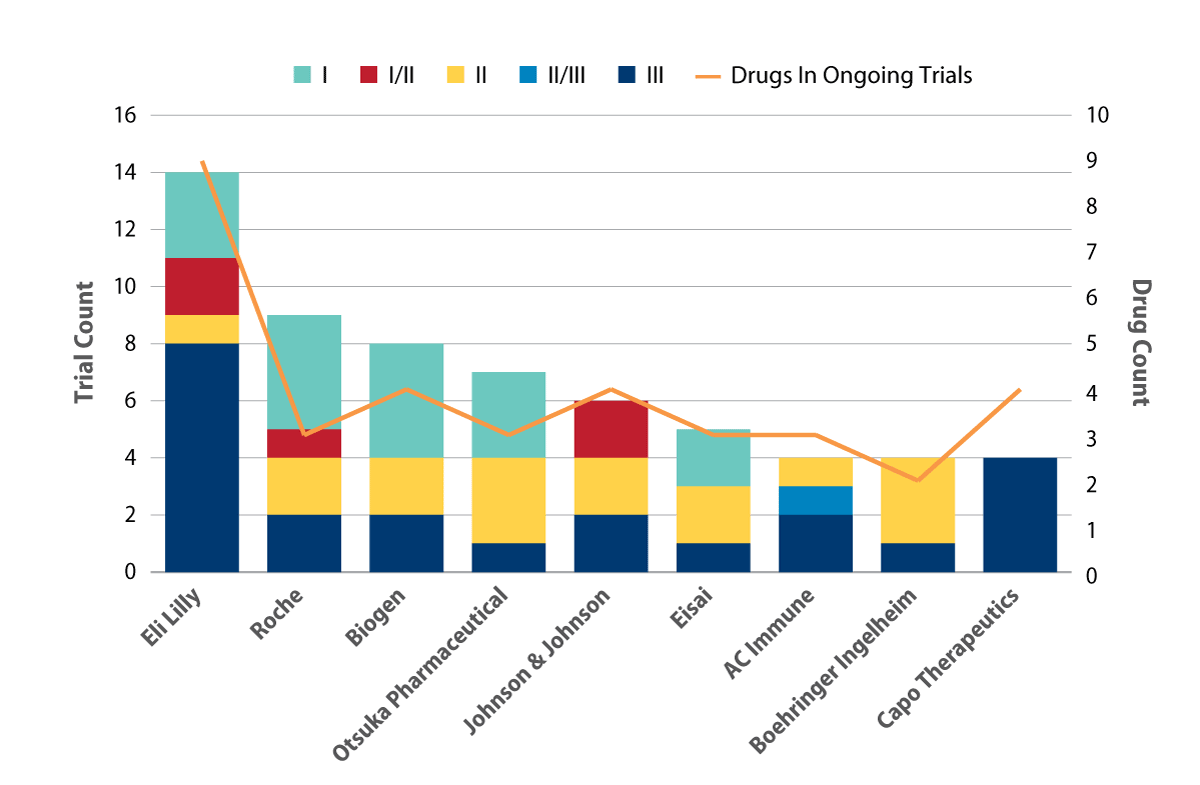
Focusing on the top industry sponsors, the key players are a mixture of top 20 pharma companies, based on annual revenue, as well as other smaller companies (see Exhibit 3). This cohort is responsible for a total of 53 trials, evaluating 31 different drug candidates. The beta-amyloid protein persists as a major target for AD with eight different beta-amyloid protein antagonists, targeting the protein directly, included in 21 trials. Another mechanism of action (MOA) targeting the amyloid pathway is the beta-site amyloid precursor protein cleaving enzyme (BACE) inhibitor, also known as the secretase beta inhibitor. This is the second most common MOA by both drug and ongoing trial count – five BACE inhibitors are being evaluated in 12 ongoing AD trials.
Lilly is the leading sponsor with 14 trials involving nine different drugs, followed by Roche (nine trials for three drugs). The bulk of Lilly’s activity falls in Phase I (eight trials) while the remaining studies are primarily late-stage evaluations of a BACE inhibitor, lanabecestat, and the previously mentioned beta amyloid protein antagonist, solanezumab.
Roche, on the other hand, has nearly half its trials in Phase III, splitting activity between two beta amyloid protein antagonists: crenezumab and gantenerumab. Crenezumab is also included in a Phase II prevention trial, partnered with AC Immune, which targets PSEN1 E280A mutation carriers who are in the preclinical phase of AD.
Other active top 20 pharma companies include Johnson & Johnson (J&J; six trials for three drugs) and Boehringer Ingelheim (four trials for two drugs). Meanwhile, smaller players with robust ongoing activity include Otsuka Pharmaceutical Co. Ltd., AC Immune SA and Capo Therapeutics. Biogen Inc. and Eisai Co. Ltd. are also key players, with four collaborative trials ongoing between the two companies. This partnership started in 2014 to develop and commercialize two of Eisai’s candidates, elenbecestat (E-2609) and BAN-2401. Both are in one closed Phase II trial each, with projected primary completion dates in 2018. Elenbecestat is also in two recruiting Phase III studies that initiated in 4Q 2016 and are both expected to complete in 2020.
Exhibit 3. Top Industry Sponsors Of Ongoing Trials
On The Horizon
In addition to this ongoing clinical research, 90 further trials are waiting in the wings and remain in planning phases. Sponsors for planned activity include a familiar cast of players, such as Biogen, J&J, Lilly, and Roche, as well as companies looking to increase their involvement. Although companies have faced setbacks and unexpected results within the field, the industry seemingly remains committed to identify effective drug interventions to combat, or prevent, this devastating disease.