Merck & Co. Strengthening Its Strong Position In Immuno-Oncology
Executive Summary
Additional data presented at ASCO support earlier use of Keytruda in key settings like first-line lung and bladder cancers and approval in new indications like triple-negative breast and gastric cancers. Competitor setbacks also clear the stage for Merck.
This year's American Society of Clinical Oncology meeting left Merck & Co. Inc. in an even more attractive immuno-oncology position than before – with additional data helping to secure the lead for its PD-1 inhibitor Keytruda in key indications like lung and bladder cancer, where competitors have fallen back, as well as promising debuts in new tumor types like breast and gastric tumors.
Merck stressed during a June 5 investor briefing at ASCO that it is working to establish Keytruda as a foundation in cancer treatment. It presented 50 abstracts for Keytruda across 16 different types of tumors at the conference, which was held from June 2 to 6 in Chicago.
Ongoing Registrational Studies Of Keytruda
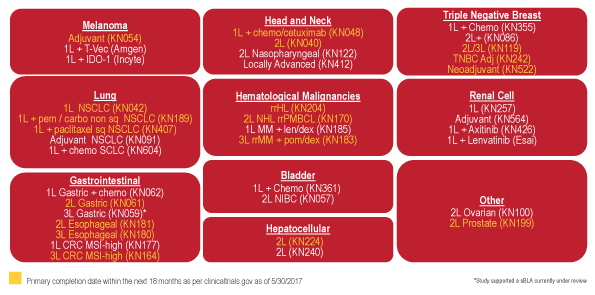
Source: Merck ASCO 2017 briefing
More than 500 trials of Keytruda in 30 tumor types and 300 combination studies are ongoing. About forty registrational studies are under way in numerous lines of therapy and tumor types – including liver cancer and triple-negative breast cancer (TNBC).
Credit Suisse analyst Vamil Divan deemed Merck's ASCO briefing "a tidy summary of the progress [Merck] has made in oncology over the past several years and … the significant opportunity ahead of the company over the next several years."
In a June 5 note, Divan expressed confidence that Keytruda and the IO opportunity is enough to carry the company. "Keytruda has now shown activity in more than 20 tumor types, has regulatory approvals in 10 different indications and has ~40 registration-enabling studies ongoing, driving our view that Keytruda can continue to drive the story in a company where we acknowledge the base business is facing pressure and where the non-oncology pipeline is not as robust as we would prefer."
Divan wasn't alone in his positive assessment. Leerink analyst Seamus Fernandez concluded after the event – in a note titled "How Broad Are Keytruda's Shoulders?" – that "if there is one theme that continues to resonate at [Merck], it is that science-driven clinical execution has been the foundation of Keytruda's success and this is likely to continue for the foreseeable future."
Merck notes that Keytruda (pembrolizumab) is emerging in three waves across tumor types, starting with melanoma and NSCLC (see table). It is currently approved across different lines of therapy for lung cancer, melanoma, classical Hodgkin lymphoma, head and neck squamous cell cancer, bladder cancer and microsatellite instability-high cancers.
|
Keytruda Advancing In Three Waves |
||
|
First wave |
Second wave |
Third wave |
|
Melanoma [X] NSCLC [X] |
Head and neck [X] Bladder [X] Gastric TNBC Colorectal [X] Hodgkin lymphoma [X] MSI-high [X] |
Esophageal Renal Ovarian Prostate Multiple myeloma Non-Hodgkin lymphoma Hepatocellular Other thoracic malignancies Gynecological malignancies Rare tumors |
|
Source: Merck ASCO 2017 investor briefing |
||
Looking Ahead To New Cancers
The company announced in late May that FDA has accepted yet another supplemental filing in gastric cancer after two or more prior lines of therapy. The filing is under priority review. At ASCO, Merck presented data from the heavily pretreated single arm "Cohort 1" of the KEYNOTE-059 study supporting the filing. The objective response rate (ORR) was 11.6% overall, with a 15.5% ORR in those positive for expression of the PD-L1 biomarker and 6.4% ORR in PD-L1-negative patients.
Phase II data from the I-SPY study at ASCO suggest that Keytruda with chemotherapy in the neoadjuvant setting would have a very high probability of succeeding in Phase III studies in certain types of high-risk breast cancer, including triple-negative breast cancer. Among other promising findings, I-SPY researchers noted that there was a tripling of the rate of pathological complete response, a surrogate marker for overall survival, for Keytruda and chemo versus chemo alone. (Also see "Merck's Keytruda Offers Hope And Risk In Early Breast Cancer" - Scrip, 6 Jun, 2017.) Merck is running five studies that it views as registration-enabling of Keytruda in breast cancer.
In addition to moving into new tumor types, Merck is benefiting from early leads in certain markets where others are failing – chiefly Bristol-Myers Squibb Co.'s PD-1 inhibitor Opdivo (nivolumab) failing in first-line non-small cell lung cancer in last year's CheckMate 026 study and more recently, Roche's PD-L1 inhibitor Tecentriq (atezolizumab) failing in its confirmatory bladder cancer trial. (Also see "Bladder Cancer Market Wide Open As Tecentriq Fails Confirmatory 2L Trial" - Scrip, 10 May, 2017.)
Building Evidence In Lung Cancer
Lung cancer is the most valuable indications for PD-1/L1 immunotherapies and Keytruda has gained the first and only approval as a monotherapy in first-line NSCLC in October 2016 and the first and only approval of a combination in the first-line setting this May.
Even with competition looming from PD-1/CTLA-4 combination studies – "soon" for AstraZeneca's MYSTIC trial of durvalumab/tremelimumab and next year for Bristol's Opdivo/Yervoy pairing in first-line lung – the early lead Merck has with its cheaper Keytruda/chemo combo could be a significant advantage.
"The strong foundation [Merck] is building in the space now will benefit them over time," Credit Suisse's Divan said, which the analyst thinks is underappreciated.
The combination of Keytruda with Eli Lilly & Co.'s chemotherapy Alimta (pemetrexed) and carboplatin chemotherapy received accelerated approval for first-line, non-squamous, metastatic NSCLC, about one quarter of the first-line market, based on ORR data. (Also see "Keytruda/Chemo Combo Approval Means Merck Holds Crown, For Now" - Scrip, 10 May, 2017.) The confirmatory study for the filing is the Phase III KEYNOTE-089 study of Keytruda in first-line NSCLC, which is due to report later this year.
At ASCO, Merck released some positive updates that bolster Keytruda's position in lung cancer. In KEYNOTE-024, the pivotal trial for the monotherapy approval in first-line NSCLC with at least 50% PD-L1 expression, the company reported an overall survival benefit continued through the two-year point.
Merck also presented an update on the "G1" cohort of the KEYNOTE-021 study that supported the Keytruda/chemo combo approval. The ORR in the trial was 56.7% for the combination with Keytruda versus 30.2% for the comparator. An overall survival benefit is starting to emerge – the survival rates at nine months and 12 months, respectively, were 84.6% and 76% for the Keytruda arm vs. 82.3% and 69.3% for the chemo combo alone, a non-significant improvement, Merck reported.
During the investor briefing, Merck Research Labs President Roger Perlmutter noted that there had been substantial crossover of 75% in the study and that despite this, the company is beginning to see a trend toward improvement in overall survival.
"Importantly, these responses seem to be durable, as they typically are with Keytruda treatment," Perlmutter said.
Though the combination is approved, many lung cancer specialists are "disinclined to embrace it without an overall survival benefit," Howard (Jack) West, director of the thoracic oncology program at the Swedish Cancer Institute in Seattle, tweeted after the meeting. "Will a non-significant trend move us from 'can' to 'should' pursue this?" he asked.
In an interview, Merck's Roy Baynes, senior vice president of global clinical development at Merck, noted that the study is the first randomized trial to improve progression-free survival in 35 years and showed "quite striking efficacy." Furthermore, a big confirmatory study is coming, he added.
Credit Suisse's Divan sees the commercial opportunity for Keytruda expanding in lung cancer.
"In NSCLC, Keytruda is already capturing the bulk of patients with tumors that have high levels of PD-L1 expression. With the recent FDA approval for Keytruda + Alimta across all of 1L non-squamous NSCLC and, following data at ASCO that showed a trend in overall survival in the KN-021G study, we expect that combination to gain further traction as we move into 2H 2017," Divan said.
Frank Clyburn, president of Merck's global oncology unit, said during the company's ASCO briefing that overall, early feedback in the marketplace about the combination has been "very positive" and that physicians are comfortable using it. Merck also has received some positive feedback on the updated OS curves for the 21G cohort, the exec said.
Another notable release in lung cancer at the meeting was data for Keytruda in combination with partner Incyte Corp.'s epacadostat, an inhibitor of the up-and-coming immuno-oncology target indoleamine 2,3-dioxygenase (IDO1), an enzyme that plays an important part in immune response, in the Phase I/II ECHO-202 study. (Also see "Scrip's Rough Guide To IDO" - Scrip, 18 May, 2017.)
A cohort of 40 patients with NSCLC showed an objective response rate of 35%, including complete responses in two patients (5%). Responses were demonstrated regardless of the level of PD-L1 expression, the company said. Results for other tumor types were in line with expectations, though data for the tough-to-treat triple-negative breast cancer and ovarian cancer were disappointing (see table).
Phase I/II ECHO-202 Results: Keytruda/Epacadostat Data, IO Naïve Population | |||||
Tumor type & number of patients | Squamous cell cancer of the head and neck (n=38) | Non-small cell lung cancer (n=40) | Renal cell carcinoma (n=30) | Bladder cancer (n=40) | Triple negative breast cancer (n=39); ovarian cancer (n=37) |
Abstract number | #6010 | #9014 | #4515 | #4503 | #1103 |
Objective response rate | 34% overall ORR; 39% after 1-2 prior lines of therapy, 14% ≥3 prior lines. | 35% overall ORR; 39% after 0-2 prior lines therapy | 33% ORR overall; 47% after 0-1 prior lines, 9% ≥2 prior lines | 35% ORR overall; 38% after 0-1 prior lines, 25% after ≥2 prior lines | TNBC: 10% ORR overall, 12% after ≤2 prior lines, 9% ≥3 prior lines Ovarian: 8% ORR overall; 13% after ≤2 prior lines therapy,7% ≥3 prior treatments |
Treatment-related adverse events (Abstract #3012): In an updated pooled analysis, TRAEs in 67% of 294 patients. Most common: fatigue (29%), rash (17%), nausea (11%) and pruritus (10%). Grade ≥3 TRAEs in 18%. Most common: increased lipase (asymptomatic) (4%) and rash (3%). TRAE-related discontinuation rate: 4%. | |||||
Sources: Adapted from presentation by Siwen Hu-Lieskovan (UCLA) at ASCO 2017, company releases | |||||
Bladder Data Hold Up Over Time
Survival data presented for Keytruda in bladder cancer is also encouraging. Keytruda became the first PD-1/L1 inhibitor to secure full approval in second-line metastatic urothelial cancer after platinum-based chemotherapy, having proven a survival benefit over chemotherapy in this setting. (Also see "Kalydeco Expands Indication Without Clinical Data; Keytruda Is Latest Bladder Cancer Approval" - Pink Sheet, 21 May, 2017.)
All of the other leading PD-1/L1 drugs approved for second-line bladder cancer hold accelerated approvals based on response rate data – Roche's Tecentriq(atezolizumab), Bristol's Opdivo (nivolumab), AstraZeneca PLC's Imfinzi (durvalumab), and Merck KGAA/Pfizer Inc.'s Bavencio (avelumab). And Roche's Tecentriq recently failed to show an overall survival benefit in a confirmatory trial in second-line bladder cancer. (Also see "Could Tecentriq's Bladder Cancer Setback Be A Class Effect?" - Scrip, 12 May, 2017.)
Keytruda also holds the only approval in the first-line setting for cisplatin-ineligible metastatic bladder cancer.
At ASCO, Merck presented updated data from the Phase III KEYNOTE-045 study that supported the second-line approval. In the study, median overall survival was 10.3 months for Keytruda monotherapy vs. 7.5 months for physician's choice of chemo, a significant 27% reduction in risk. Investigators reported in a poster study at the meeting that Keytruda's survival advantage held with longer follow up – at 12 months, 44% were alive in the Keytruda arm versus 30.2%, and at 18 months, 36.1% were alive versus 20.5%.
Memorial Sloan Kettering oncologist Jonathan Rosenberg noted in a discussion of the poster that the longer follow-up of survival data confirmed the initial analysis from the trial and that objective responses occurred rapidly and were durable. Safety and tolerability also clearly favor pembrolizumab over second-line and third-line chemotherapy, he said.
Keytruda is the only PD-1/L1 agent to have a "Category 1" recommendation, meaning the highest level of evidence, from the National Comprehensive Cancer Network in this indication. Rosenberg said that Keytruda is now the standard of care in second-line metastatic bladder cancer, though the field is moving rapidly and is subject to change (see table).
"Many of the newer agents have been approved in bladder, but we feel very good about our data. And in fact … one of our key customers just announced that they are going to add Keytruda for bladder into their regimens based on the strength of KEYNOTE-045," Merck's Clyburn said during the analyst briefing.
The combination of Incyte's epacadostat with Keytruda represents a new approach to the tumor type. In the ECHO-202 study, the ORR was 35% in 40 patients. Rosenberg noted that the population was rather lightly pretreated – 80% had one or fewer prior regimens in the metastatic setting – but that was also the case in Keytruda's KEYNOTE-045 and Opdivo's CheckMate 275 monotherapy studies. In those trials, the ORR was 19.6% and 21%, respectively.
"We can be at least somewhat comfortable that the objective response rate [for the combination] is a real thing. Certainly, this is worthy of further evaluation," Rosenberg said.
Advanced Bladder Cancer Treatment Algorithm | |||
Disease state | Context | Category 1 Evidence | Standard Options |
Metastatic, no prior chemotherapy | Cisplatin-eligible | Cisplatin-based combination chemotherapy | |
Metastatic, no prior chemotherapy | Cisplatin-ineligible | Atezolizumab, pembrolizumab, gemcitabine-carboplatin, single agent chemotherapy | |
Metastatic, prior platinum chemotherapy or relapse within one year of perioperative cisplatin | Pembrolizumab | Atezolizumab, nivolumab, durvalumab, avelumab | |
Metastatic, prior immunotherapy | Taxane chemotherapy, vinflunine (in EU) | ||
Source: J Rosenberg, ASCO 2017 | |||
What Else For Merck
Leerink's Fernandez noted that the two biggest questions from investors are:
- "How broad and early can Keytruda go?"
- "When will a fully owned [Merck] combination emerge?"
More than half of the 500 trials ongoing with Keytruda involve combinations, Perlmutter told the call. There are "lots of opportunities to combine it with other therapies using a very scientific approach to try and identify those things that will improve lymphocyte activation, improve priming, improve durability and increase the representation of recognizable epitopes on the tumor cells the immune system must attack, the exec explained.
Merck does have a stable of internal partners in the clinic, including direct immune agonists like GITR and STING, and approaches to affect the tumor microenvironment (see table below).
But the nearest term combinations are likely to come from partnerships like the IDO alliance with Incyte or vaccine combinations, such as an RNA-based vaccine program partnered with Moderna Therapeutics LLC.[See Deal]
Merck announced a setback for one of its combination programs June 12, when it halted enrollment in two Phase III trials of Keytruda plus an immunomodulator from Celgene Corp. (Pomalyst and Revlimid) in multiple myeloma due to reports of higher deaths in patients on combination therapy. (Also see "Death Excess Gives Pause To Merck & Co Keytruda MM Phase III Studies" - Scrip, 13 Jun, 2017.)
Pushed as to why Merck hasn't emphasized advancing combinations in the same way Bristol and Roche have, Perlmutter responded that Merck's strategy is based on better understanding why patients aren't responding to find the best way to broaden that response base – different approaches for different situations should yield the best responses. "As we begin to evaluate patients and to assign them systematically to these mechanistically quite different reasons for nonresponsiveness, we'll understand how best to apply new mechanisms," he said. "And we're exploring new mechanisms that address all of those different areas."
Immediately following ASCO, Merck advanced its own CTLA-4 combination trial into Phase I, in lung and other tumor types. The trial is pairing Keytruda with Merck's own internal candidate, MK-1308, rather than Bristol's marketed Yervoy or AstraZeneca's Phase III tremelimumab – which both firms are testing with their own PD-x inhibitors.
"We have felt that if [Merck] is going to pursue CTLA-4 combo – either as a hedge, or because it genuinely believes in the opportunity – it either needs to 'go big or go home,'" Bernstein Research analyst Tim Anderson said in a June 8 note. "Unless [Merck] believes it has a differentiated product, it is difficult to justify development of this new molecule given the substantial lead-time advantage that its two competitors have in this area."
He noted that Merck execs have previously indicated that there might be some differentiation – but as that would be based on preclinical/mechanistic studies "the supporting evidence behind this claim is weak at the moment." Instead, Anderson suggested Merck should simultaneously pursue a Keytruda/Yervoy trial, not necessarily a full registrational trial but something that would be "practice enabling" and build physician comfort "mixing and matching" products. He indicated Merck management has been receptive in the past but may have paused due to lackluster combination data emerging – like Bristol's CheckMate-067 data in melanoma at the American Association of Cancer Research meeting or the CheckMate-012 data at ASCO.
During the ASCO briefing, Perlmutter weighed in on the CTLA-4/PD-1 pairings: "There's reason to believe that you can see some improvement as a result of combining the two," but "the question is sort of is the juice worth the squeeze?" – i.e., does the added benefit justify the increased toxicity. There are "slightly different properties" with Merck's CTLA-4 agent, he added. "We're systematically trying to understand how best to use the combination together because we're concerned about the toxicity."
The landscape will become clearer as the AstraZeneca and Bristol trials report out, but in the meantime Merck is concentrating on making the most of the chemo combo opportunity and steadily building its own mechanistically based combinations.
As Bernstein's Anderson concluded, "the long-term IO landscape may always feel like it could shift again as newer approaches (e.g. IDO combination, CEA-CD3 bispecific) advance into Phase III."
Pipeline Focused On Finding Keytruda Partners
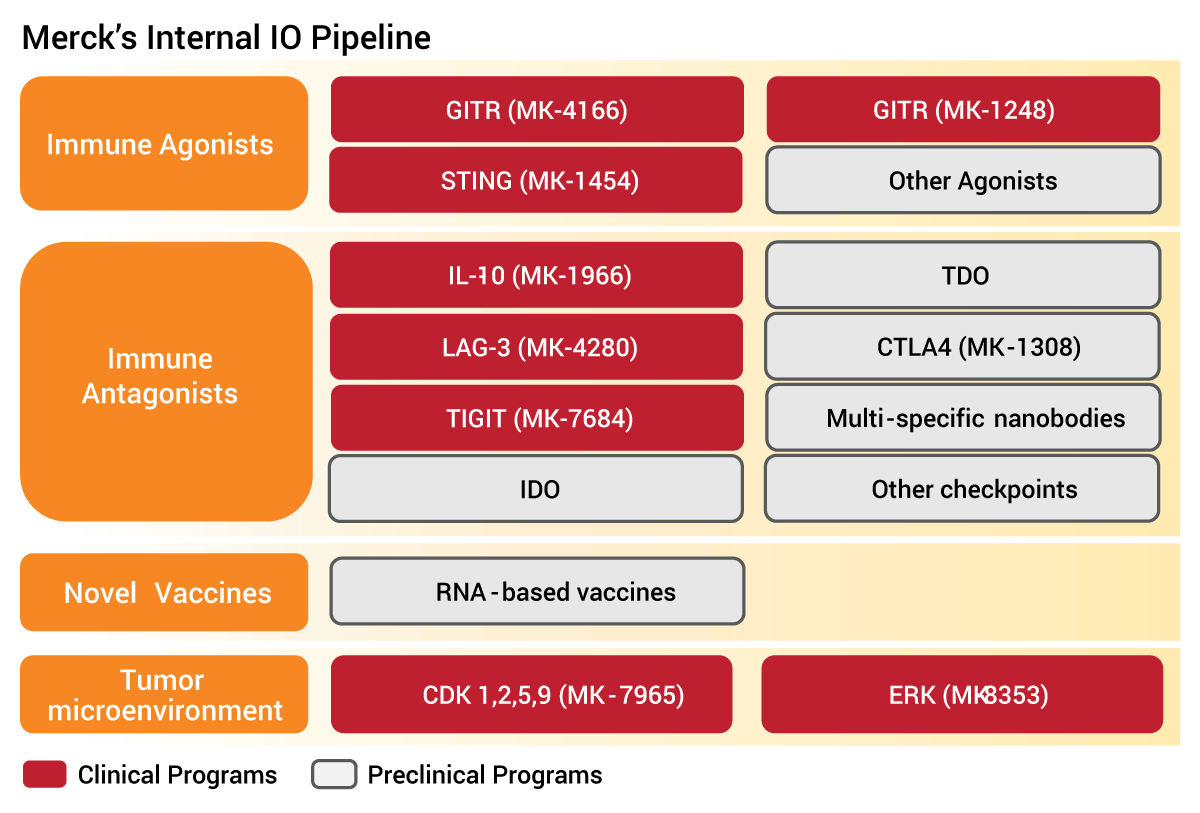
Source: Merck ASCO 2017 briefing