AbbVie Makes Further Inroads In Oncology As Humira Loss Looms
Executive Summary
New data position BCL-2 inhibitor Venclexta, partnered with Roche, for roles in multiple myeloma and acute myeloid leukemia, among other indications beyond chronic lymphocytic leukemia.
AbbVie Inc. is making inroads in oncology, expanding the potential reach of its first approved hematological cancer drugs Imbruvica and Venclexta while advancing new pipeline candidates ahead of patent expirations for the blockbuster tumor necrosis factor inhibitor Humira.
Oncology has represented a newer, promising growth area for AbbVie, and the company has been investing heavily in the pipeline for this therapeutic area through partnerships, in-house development and ambitious expansion plans for the first approved drugs. (Also see "Oncology Deal Spree To Continue For AbbVie – Early Cancer Pipeline Outweighs Other Disease R&D" - Scrip, 23 Sep, 2016.) Growth in areas new and old are important for AbbVie as the company prepares to face sales losses due to biosimilar products competing with Humira (adalimumab), the top-selling treatment for arthritis and other autoimmune conditions that generated sales of $6.43bn in this year’s third quarter. (Also see "Another Strong Quarter For AbbVie, But How Long Will Good Times Last?" - Scrip, 29 Jul, 2016.)
It’s not clear when biosimilars for the TNF inhibitor will hit the market, even after US FDA approval in September for Amjevita (adalimumab-atto), Amgen Inc.'s biosimilar version of Humira. But while a patent dispute between AbbVie and Amgen will delay Amjevita’s launch beyond 2017, competition clearly is nipping at Humira’s heels, and AbbVie is betting big on cancer drugs to help fill the product’s shoes. (Also see "Biosimilars: Sandoz Pegfilgrastim Review, Amgen Adalimumab Launch Extended To 2018" - Pink Sheet, 28 Oct, 2016.)
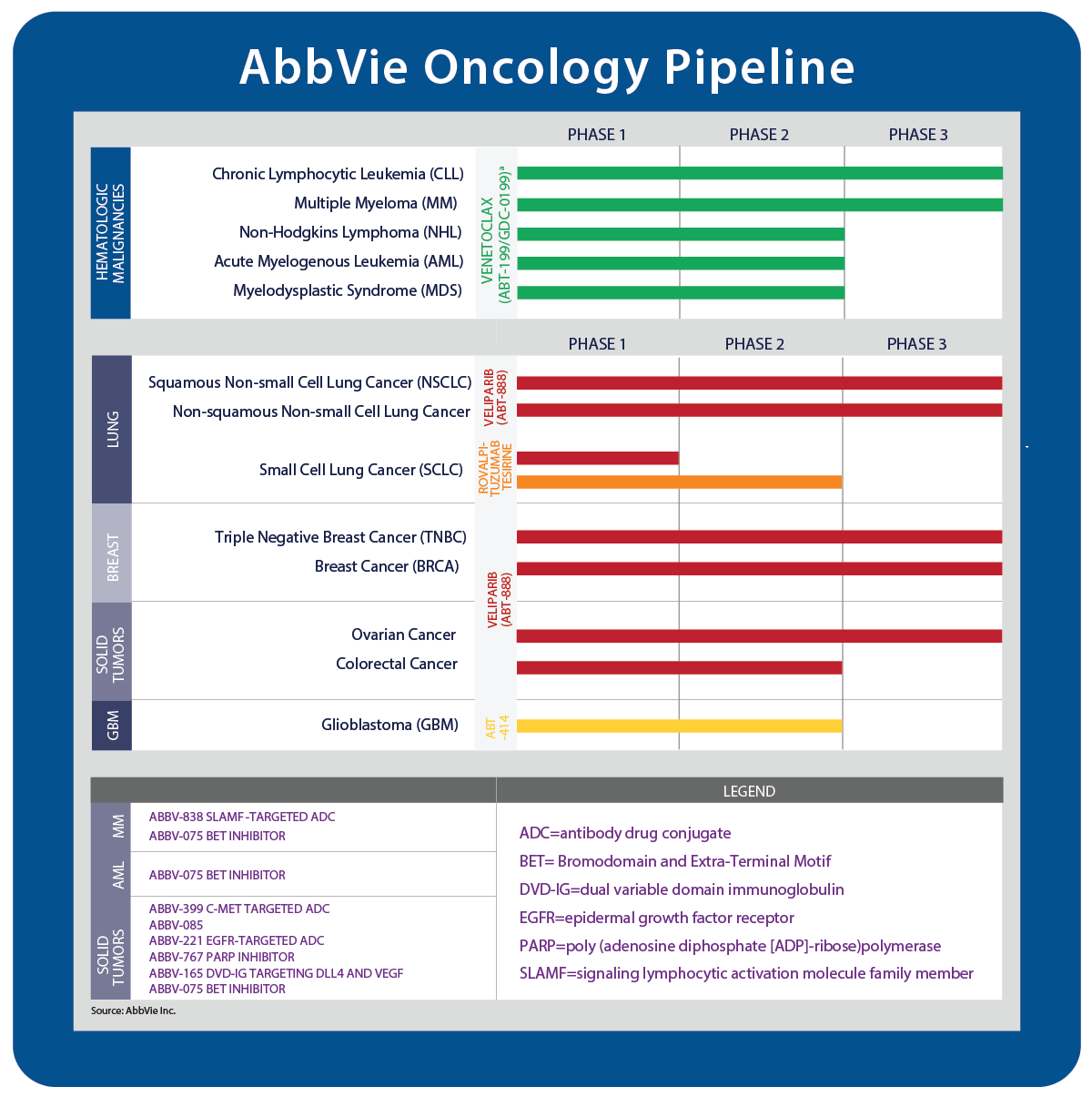
Oncology is one of the company's most active areas of development. Some 13 drugs are in clinical development for 22 different indications, including hematological malignancies and solid tumors.
AbbVie’s BTK inhibitor Imbruvica (ibrutinib), which is partnered with Johnson & Johnson unit Janssen Biotech Inc., has proven to be a big success, with approvals in chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), Waldenström's macroglobulinemia, and many more indications on the way.
The partners presented impressive long-term data for Imbruvica in CLL during the American Society of Hematology (ASH) annual meeting from Dec. 3-6 in San Diego, as well as Phase I/II data showing a 67% objective response rate in graft-versus-host disease, a condition that may occur if donor cells attack the body after stem cell and bone marrow transplants. (Also see "AbbVie/J&J's Imbruvica On Track For New Indication In Graft-Versus-Host Disease" - Scrip, 6 Dec, 2016.)
AbbVie reported sales of $659m for Imbruvica in 2015 and partner J&J reported $689m. Each of seven new filings in coming years will bring another $500m in revenue, Abbvie estimates.
Imbruvica is under FDA review for marginal zone lymphoma; in addition to graft-versus-host disease, it is in Phase III for indolent non-Hodgkin lymphoma (iNHL), follicular lymphoma (FL), B-cell NHL and diffuse large B-cell lymphoma (DLBCL). Imbruvica also is in Phase II for acute myeloid leukemia (AML) and multiple myeloma.
Venclexta's Promise In Myeloma
AbbVie also has high hopes for its BCL-2 inhibitor venetoclax, partnered with Genentech Inc., which was approved for second-line CLL with 17p deletions in April in the US, where it is marketed as Venclexta. (Also see "Venclexta Win Broadens AbbVie/Genentech CLL Positions " - Scrip, 12 Apr, 2016.)
Venetoclax was approved this month in Europe, where is branded as Venclyxto, for second-line treatment of CLL with 17p deletions. (Also see "Venclyxto Gets EU Nod – But Won't Be A Humira Replacement For AbbVie Yet" - Scrip, 14 Oct, 2016.) Only about 10% of treatment naïve CLL patients have 17p deletions, but the indication is a gateway into much broader use, although it’s dominated by Imbruvica.
Chief Financial Officer Bill Chase acknowledged during AbbVie’s third quarter earnings call Oct. 28 that the market for relapsed/refractory CLL with the 17p deletions was small – worth about $300m in sales – and Imbruvica has a strong leadership position with more than a 50% share of that market.
Furthermore, new late-stage competitors in CLL are on the near horizon – AstraZeneca PLC/Merck & Co. Inc./Acerta Pharma BV's BTK inhibitoracalabrutinibandTG Therapeutics Inc.'s anti-CD20 ublituximab.
Chase described venetoclax as an asset that offers significant growth potential over the longer term, with expansion into earlier lines of therapy and other hematologic malignancies. For instance, AbbVie is looking forward to results from the MURANO study, which tests venetoclax with Roche's Rituxan (rituximab) in second-line CLL in the first half of 2017 to support broader labeling.
Data for venetoclax in multiple myeloma generated buzz at the ASH annual meeting. In a Phase I study of relapsed/refractory myeloma with the t(11;14) translocation, the most common chromosomal translocation in multiple myeloma, the objective response rate with Venclexta as a single agent was 40%, almost twice as high as the response in the overall population.
Gary Gordon, vice president of oncology development at AbbVie, said in an interview that this could be one of the first applications of biomarkers in selecting multiple myeloma patients for treatment.
"These results are particularly striking given that patients in this study were heavily pre-treated with a median of five prior therapies. Subsequent biomarker analysis further revealed that patients in this subpopulation with high BCL-2 to BCL-XL ratio had the highest response rates. This is significant in that it essentially identifies a subset of multiple myeloma patients that would most benefit from Venclexta monotherapy," Biomedtracker analyst Dustin Phan said.
In a separate Phase Ib study of Venclexta with Takeda Pharmaceutical Co. Ltd.'s flagship proteasome inhibitor Velcade (bortezomib) and dexamethasone in relapsed or refractory myeloma, which also was presented at the ASH meeting, the objective response rate was 38% for those who were refractory to bortezomib and 89% in patients who were not refractory to bortezomib and had previously received one to three other lines of therapy. For the whole study population, the response rate was 67%.
Gordon noted that the combination data support examining the hypothesis that proteasome inhibitors modulate MCL-1 levels and enhance sensitivity to venetoclax as a BCL-2 inhibitor
"The drastic difference in response between these bortezomib-refractory and bortezomib non-refractory patient subgroups is not surprising given that patients who fail to respond to a particular drug are unlikely to benefit from additional administration. However, the fact that some bortezomib-refractory patients responded to the combination suggests that additional Venclexta may have helped overcome bortezomib resistance," Biomedtracker's Phan said.
Barclays analyst Geoff Meacham in a Dec. 6 note said that patients with high expression of the BCL-2 gene had an ORR of 94%, versus 59% for lower expression, plus improved time to progression.
AbbVie is running a registrational study of Venclexta with Velcade and dexamethasone in relapsed/refractory multiple myeloma (RRMM) patients who are sensitive or naïve to proteasome inhibitors and who have had from one to three prior lines of treatment. The primary completion date for the trial is December 2019.
"While these data support the ongoing Phase III trial in RRMM patients, we see limitations, especially with BCL-2 expression and whether or not a patient is refractory to bortezomib," Meacham said.
Nevertheless, the analyst said novel combinations seem to work well in prolonging progression-free survival and that he sees potential for the use of Venclexta as well as Amgen's proteasome inhibitor Kyprolis (carfilzomib) in various combinations for relapsed/refractory myeloma.
Moving In On AML
AbbVie also presented data for Venclexta with low-dose cytarabine (LDAC) in a Phase I/II study of 61 elderly patients with the tough to treat acute myeloid leukemia (AML). Investigators reported that the ORR was 61% and that side effects were manageable.
"The notion of using a BCL-2 inhibitor for elderly AML is a potentially differentiated approach given the population’s more limited options, and we are encouraged by AbbVie’s early results combining Venclexta plus LDAC. The ORR … in all patients was 61% (37/61, median age=74) and 70% (21/30) in those patients above age 75, which compares favorably with historical LDAC results in the teens range," Meacham commented.
AbbVie started a Phase III study of Venclexta with azacitidine versus azacitidine alone in treatment-naïve elderly patients with AML who are ineligible for standard induction therapy in December.
Veliparib Provides Solid Tumor Opportunity
AbbVie presented mid-stage data for another asset now in Phase III – the PARP inhibitor veliparib – at the San Antonio Breast Cancer Symposium on December 7.
The Phase II study tested veliparib in combination with different kinds of chemotherapy in 290 breast cancer patients with BRCA1 and BRCA2 mutations. Although patients taking veliparib with carboplatin and paclitaxel chemotherapy had a significantly better response rate compared to those taking carboplatin and paclitaxel with placebo (77.8% vs. 61.3%), progression-free survival and overall survival were not significantly improved compared to the control group. However, toxicity was also not significantly increased in the arm that included veliparib as part of combination therapy.
AbbVie notes that the study was not powered well enough to show a difference in progression-free survival and the company is now running the Phase III BROCADE study, which tests veliparib with carboplatin and paclitaxel in HER2-negative metastatic BRCA-associated breast cancer.
AbbVie is planning to differentiate veliparib from other PARP inhibitors on the basis that it is a potent inhibitor of PARP, it combines well with multiple chemotherapies – particularly platinum chemotherapies – without dramatic increases in toxicity, and it penetrates the central nervous system, Gordon said.
Veliparib also is in Phase III for squamous non-small cell lung cancer (NSCLC), non-squamous NSCLC, triple-negative breast cancer and ovarian cancer (see chart below).