Biotech Investors Eye Early-Stage Venture Vacuum
This article was originally published in Start Up
Executive Summary
Data compiled and analyzed by the Biotechnology Industry Organization and discussed at its recent CEO & Investor meeting in New York begs the question: why aren’t more new biotechs being started when investor and acquirer demand is so high?
The broad renaissance in biotech over the past few years has not included a commensurate bump in the number of new companies getting funded. This disconnect between supply and the (for the moment) insatiable demand of the public markets and pharmaceutical acquirers for new biotech assets creates an imbalance that endangers a sustainable long-term boom.
“The number of new companies is one metric that doesn’t seem to have bounced back up,” Jonathan Leff, partner, Deerfield Management, and chairman of the Deerfield Institute said during a panel discussion at the BIO CEO & Investor Conference in New York City in February. There remains a “shortage of capital” among venture groups that start new biotechs, and too few firms that specialize in doing so, Leff said.
The discussion was sparked by a report released by the Biotechnology Industry Organization February 9, which detailed where venture investors were placing their bets, in terms of disease category, stage of development, and along an innovation spectrum. Unsurprisingly, oncology and the related immuno-oncology area have drawn the most venture money – in each of the 10 years (2004–2013) evaluated. (See Exhibit 1.) (See (Also see "Venture Funding Continues Upward Climb, But On The Backs Of Fewer Investors – BIO Report" - Pink Sheet, 9 Feb, 2015.).) The report also found that although the total cash flowing into Series A rounds has risen for four straight years up to and including 2013, since the financial crisis in 2008, fewer investors were creating fewer new companies.
Exhibit 1
Total Venture Funding By Disease Category, 2004-2013
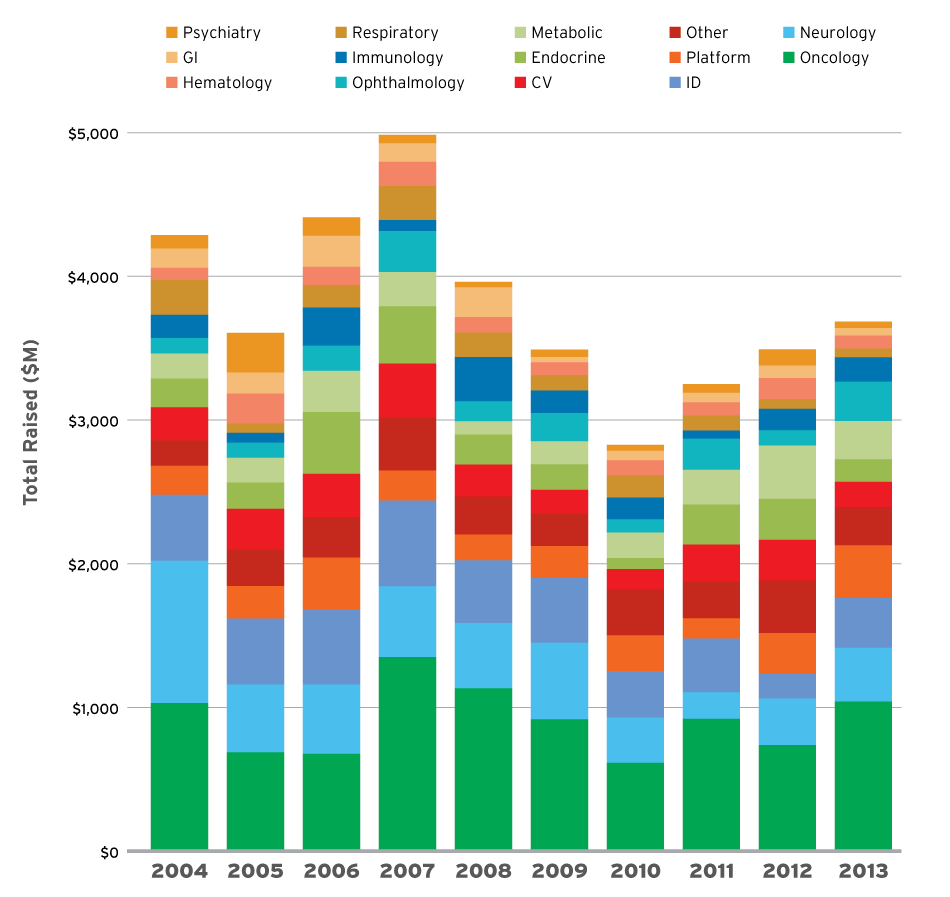
SOURCES: Informa's Strategic Transactions; BioCentury's BCIQ; EvaluatePharma; ThomsonReuters' ThomsonONE; BIO analysis
The investors who’ve remained among biotech’s staunchest backers of early-stage innovation and have continued to build new companies – a group that includes Atlas Venture, Third Rock Ventures, and others – have certainly reaped the rewards; the biotech bull market has moved into its fourth year, and the biotech IPO market is still booming. The fact hasn’t gone unnoticed, said Leff, who expects more investors to raise capital and move into earlier-stage investing, including company creation. Deerfield, he said, would be among those getting ready to “do the heavy lifting” required to build new companies.
Vacuums rarely exist for long, and with Deerfield and others eager to roll up their sleeves to participate in the trenches of building biotechs, this particular one may be disappearing. But then again it’s just as likely that additional cash isn’t what’s needed to fill the void. Even the tremendous influx of capital from corporate venture funds and, importantly, from crossover investors whose seemingly bottomless pockets have been instrumental to pre-IPO biotech financing over the past few years hasn’t yet fixed the biotech “supply problem.” Part of this has to do with VCs’ reluctance to back untested management teams (there’s a reason the term "serial entrepreneur" exists) and a real or perceived limit to just which scientific ideas should form the basis for a new biotech; another part comprises pharma’s shifting appetites and the regulatory headwinds in certain primary care markets that have stifled investment in those areas, effectively creating no-go areas for company formation. (The fact that there are cheap generics available to treat many primary care indications does not help matters either.) If nobody’s buying cardiovascular assets, to pick a one-time primary care stalwart, venture capitalists aren’t going to build companies to supply them.
BIO’s statistics – compiled by combining data from four venture databases including Informa’s Strategic Transactions – illuminate a significant drop-off in, for example, the endocrine space. From 2004 to 2008 VCs poured $962 million into funding novel R&D (as opposed to reformulations) at companies whose lead asset targeted type 1 or type 2 diabetes and other diseases. From 2009 to 2013, that figure dropped to $384 million. Although there was a slight uptick in “drug improvement” R&D in the endocrine space, the combined drop-off between the two periods was 31%. In another primary care-dominated market, gastrointestinal disease, the drop-off was 62%. Psychiatry and respiratory R&D saw similar declines. (See Exhibit 2.)
Exhibit 2
Changing Venture Tastes: Novel Drug R&D Shifts Focus
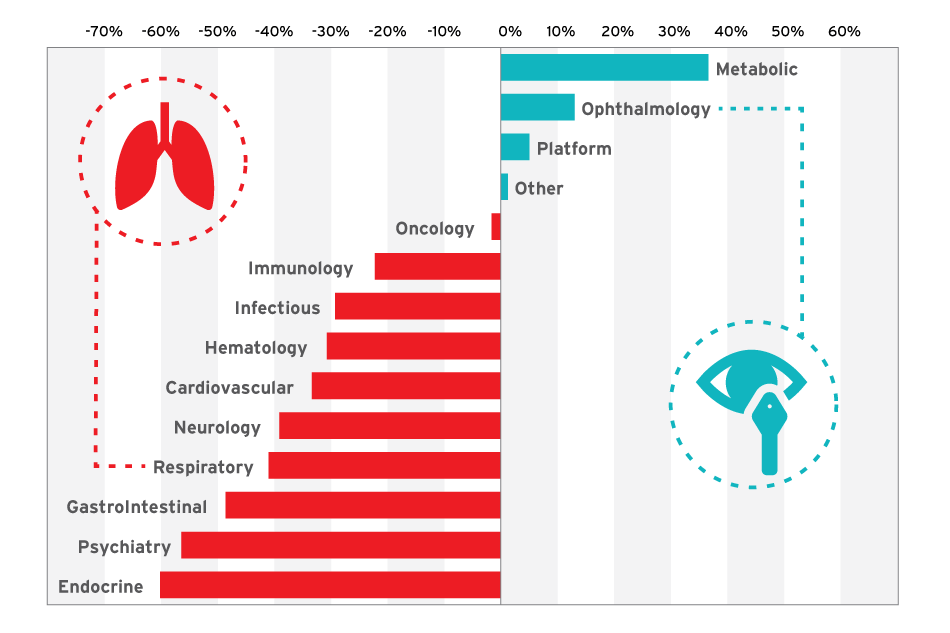
Note: Novel drug R&D venture funding by disease area, 2004-2008 vs. 2009-2013.
SOURCES: Strategic Transactions; BioCentury's BCIQ; EvaluatePharma; ThomsonReuters' ThomsonONE; BIO analysis
In the endocrine space, said Robert Kiss, managing director at JP Morgan Asset Management, which invests as a limited partner in venture funds, “the drop was [in part due to] the financial crisis, but also the Avandia controversy,” referring to the regulatory snafu that sidetracked GlaxoSmithKline PLC’s rosiglitazone. “The magnitude of the clinical trials necessary to bring the next drug like that to market were daunting.” (See (Also see "Industry To FDA: Rethink Diabetes Drug CV Safety Requirements" - Pink Sheet, 25 Sep, 2014.).)
Atlas Venture partner Jean-Francois Formela agreed, pointing out that though there were clearly macro forces at work that determined in part which therapeutic spaces rebounded after the financial crisis, “it’s quite striking that some of the large chronic disease areas,” where there’s clearly a major public health need and a huge market opportunity, “are no longer attracting VC funding” the way they used to. “It doesn’t mean a policy is wrong,” he said. “But know it might have an impact on innovation.”
The idea that onerous safety requirements in diabetes might have a chilling effect on investment is not a new one. But BIO’s data analysis was nevertheless powerful in its pre- and post-crisis approach to measuring the impact of various forces on investment in certain therapeutic spaces. At the same time, the challenges of investing in chronic diseases provide an opportunity for VCs to seek out mechanisms and targets that may not be subject to the same kinds of regulatory or safety-driven requirements. Formela pointed to Intarcia Therapeutics Inc. as a company “innovating at the frontier of existing challenges and solving those challenges with new technologies that apply to current classes of drugs.”
The supply problem is only a real problem so long as demand for biotechnology assets – from partners, acquirers, and investors – remains strong.
The supply problem is only a real problem so long as demand for biotechnology assets – from partners, acquirers, and investors – remains strong. New technology platforms, the sophisticated tools that may be helping to create what Formela called “an explosion of biology,” may help to continue to fan the flames of investor interest, even as a longitudinal glance back at most industry markers of its own health – the number of IPOs, the number of drugs approved, the amount of cash raised in follow-on public offerings – suggests that what goes up must come down.
The observers who’ve seen it all before seem to be calling for more self-awareness, in part to extend biotech’s time at the top of the cycle. Among them, Dennis Purcell from Aisling Capital. “There’s a bit of a disconnect” among private biotechnology companies right now, who think they’ll raise one or two private financing rounds “and then go public at $500 million” valuations, he said on a BIO CEO & Investor Conference panel about the 2015 market outlook. “With all of the IPOs that have come we’ll need a period of digestion.” Despite all the positive momentum, “we have to be rational. Right now I think we’re in pretty good shape."
