Korea’s Hanmi Hopes U.S. Esomeprazole Launch Will Counter Disappointing China Results
This article was originally published in PharmAsia News
Executive Summary
As Hanmi gears up for a U.S. launch of its modified version of AstraZeneca’s Nexium, it is also expanding global R&D initiatives to differentiate itself.
SEOUL – Hanmi Pharmaceutical Co. Ltd. posted disappointing results in China in Q2 despite a 19% increase in sales. The company hopes to rebound in the second half if it can launch Esomezol, an incrementally modified generic of AstraZeneca PLC blockbuster Nexium (esomeprazole), in the U.S.
“We will focus on the U.S. market for a while and plan to begin sales in the U.S. as soon as possible before the launches of generics after the end of the Nexium patent period in May 2014,” Hanmi told PharmAsia News, noting that its marketing partner Amneal Pharmaceuticals LLC will be in charge of U.S. sales. “We don’t have a clear timetable for the launches of the product in other markets beyond the U.S. market, but the FDA approval will make the entries into other countries easier.”
Hanmi filed for Esomezol in the U.S. through the 505(b)(2) pathway in October 2010 and has received tentative approval. The pathway allows sponsors to propose a limited change to a previously approved product and reference that product’s safety and effectiveness data. A product approved under this pathway would not be AB rated – meeting U.S. FDA bioequivalence standards – and thus could not be automatically substituted for the brand (Also see "Crestor Patent Settlement Allows Actavis Generic Entry In May 2016" - Pink Sheet, 25 Mar, 2013.).
Hanmi didn’t give details, but said the product would be produced in the U.S. by a contract manufacturing organization with the raw materials shipped from Hanmi in Korea.
“The modified product will be sold in the U.S. at prices lower than the original product,” Hanmi said.
Hanmi is engaged in a patent battle with AstraZeneca, which is appealing a recent ruling by the U.S. District Court for the District of New Jersey. In that judgment, Hanmi and Amneal acknowledged that two asserted patents that protect Nexium (patent numbers 5,714,504 and 5,877,192) are enforceable and valid. But the judgment also provides that Hanmi’s esomeprazole does not infringe those patents under a claim construction ruling issued by the District Court on Dec. 12, 2012. Hanmi and Amneal have not said whether they would pursue an at-risk launch as they await a final decision (Also see "AstraZeneca, Korea’s Hanmi Move To Appellate Court In Nexium Patent Dispute" - Scrip, 4 Jun, 2013.).
An Aug. 1 note from Shinhan Investment Corp. forecast Hanmi’s exports could grow 284% over the prior year to KRW 21.9 billion in the second half of this year, largely on the back of a U.S. launch of Esomezol.
Noting that Hanmi continues to invest more in R&D, Samsung Securities analyst Lee Hanyoup predicted in an Aug. 1 note to investors that Esomezol’s U.S. debut is imminent:
“The drug’s patent expires in May 2014, at which time India’s Ranbaxy plans to launch a generic version, which will lead to competition among three products. Competition will likely jump 180 days after Ranbaxy’s launch as more generics hit the market. Hanmi’s Esomezol would have an approximate first-mover advantage of eight months over such generics if it hits the U.S. market in 2H,” the Samsung analyst said.
If fully approved, Esomeprazole would be the first 505(b)(2) drug from a Korean company, and Hanmi would become the first Korean company to enter the U.S. market under the Hatch-Waxman statutory framework.
Beefing Up In China
In addition to the U.S., Hanmi has been trying to generate new growth momentum from other overseas markets including China. Its China subsidiary Beijing Hanmi Pharmaceutical Co., Ltd. produces roughly 20 types of generics and health products, including probiotic Medilac-Vita, anti-inflammatory gel ketoprofen and respiratory agent ambrocol (Also see "Suffering At Home, Korea’s Hanmi Pharmaceutical Looks Abroad" - Scrip, 11 May, 2012.).
Beijing Hanmi’s revenues grew 19% in the second quarter as sales of flagship products Mamiai, Yitanjing, and Meichangan rose 10%, 25%, and 32%, respectively. The company’s operating margin, however, slipped 3.5% year-over-year to 7%, and its operating profit plunged 20% on slower sales growth coupled with a doubling of marketing expenses, Samsung Securities’ Lee said.
Beijing Hanmi reported RMB 7.7 billion (KRW 140 billion) in sales. In a July 31 presentation to analysts, the company said it invested 8% of its China sales in R&D.
Hanmi says it is also boosting its sales efforts in China by linking its 1,000 sales reps through IT tools such as smart phones.
The firm left its full-year guidance of 30% sales growth and a 15% operating margin unchanged on expectations of its performance improving in the second half.
“We recommend investors observe whether or not the firm can overcome unfavorable surroundings (eg, stricter regulations in China) in 2H,” the analyst wrote, referring to China’s stepped up efforts to stem corruption (Also see "Putting GSK’s China Bribery Crisis In Context" - Scrip, 18 Jul, 2013.) and (Also see "A Closer Look: Retail Price Maintenance Is Latest Hurdle For Multinationals In China" - Scrip, 7 Aug, 2013.).
Although Beijing Hanmi reported disappointing earnings in Q2, Shinhan Investment Corp. said in an Aug. 1 note to investors that pediatric medicines should recover in the third quarter from a sales slump caused by weak seasonal demand.
Global R&D Expansion
Hanmi is differentiating itself by an R&D strategy that includes external collaborations with global pharmaceutical companies and an internal strategy that is moving from generics to reformulations and mixed-dose combinations to new chemical entities and biobetters.
The company’s biobetters are based on its novel LAPSCOVERY (long-acting protein/peptide discovery technology) platform. The company noted during the July 31 presentation that its platform would expand the next generation of protein therapeutics by extending half-life and improving efficacy for a lower price point. It has four Phase II molecules in its LAPSCOVERY pipeline, and three in Phase I (see chart).
|
Hanmi LAPSCOVERY Pipeline |
||
|
Candidates |
Characteristics |
Development Stage |
|
LAPS-Exendin (HM11260C) |
Long-acting exendin-4 analog |
Phase II (U.S., EU) |
|
LAPS-hGH (HM10560A) |
Long-acting hGH |
Phase II (Korea, EU) |
|
LAPS-GCSF (HM10460A) |
Long-acting G-CSF analog |
Phase II |
|
LAPS-EPO (HM10760A) |
Long-acting EPO |
Phase I |
|
LAPS-IFNα (HM10660A) |
Long-acting IFNα |
Phase II |
|
LAPS-insulin (HM12460A) |
Long-acting insulin |
Phase I |
|
Source: Hanmi Q2 presentation |
||
Hanmi also has a small molecule pipeline focused on oncology, autoimmune and metabolic disease. It has three compounds in Phase II in gastric, breast and non-small cell lung cancer, and Phase I candidates in rheumatoid arthritis, colon cancer and non-small cell lung cancer.
Hanmi’s strategy is in line with what the Korean government hopes other local companies will do – invest heavily in R&D and become global players. To that end, the Ministry of Health established a global M&A fund to expedite deals between domestic and foreign pharmaceutical companies (Also see "South Korea Health Ministry Eyes Fund To Encourage M&A" - Scrip, 15 Oct, 2012.).
Hanmi Q2 presentation
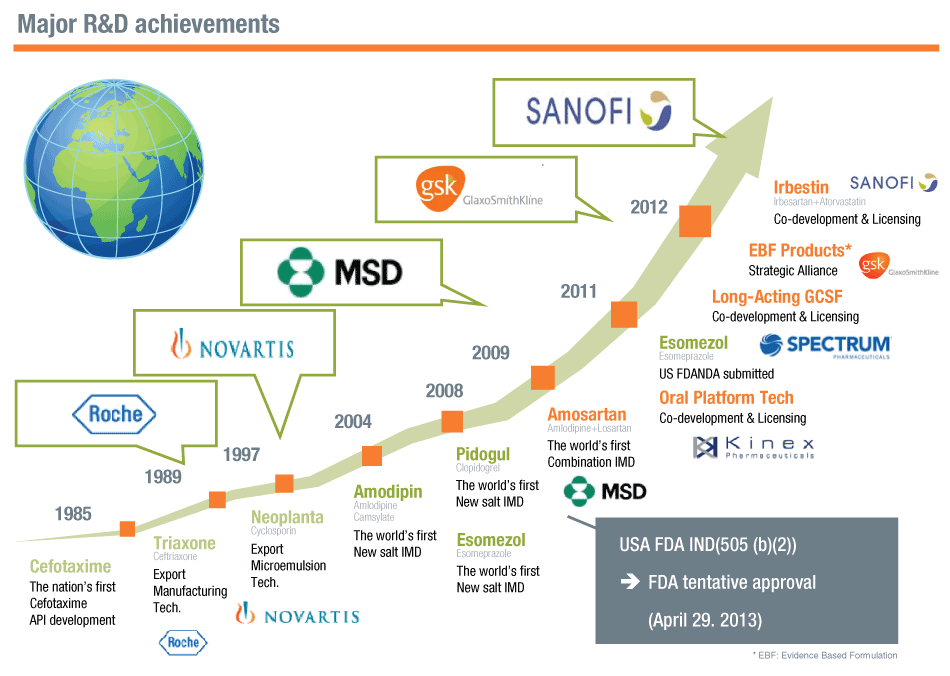
Hanmi signed a co-marketing agreement in 2012 to provide fixed-dose combination products to the French drug maker Sanofi after they are approved in Korea. The fixed-dose combination products would be sold initially in Korea in 2013, and then abroad under different brand names (Also see "Sanofi And Korea’s Hanmi Ink Co-marketing Deal That Could See Sanofi Selling Hanmi’s Irbestin Abroad" - Scrip, 23 Nov, 2012.).
Hanmi also inked a deal with GlaxoSmithKline PLC last year to co-develop what it calls evidence-based formulation products and market them worldwide. Under the deal, the two will share clinical development costs, with Hanmi in charge of formulation research, early clinical studies and manufacturing, and GSK in charge of late-stage studies and product registrations (Also see "Will GSK-Hanmi Deal Trigger Similar Tie-ups With Korean Pharmas?" - Scrip, 3 Apr, 2012.). The Korean pharma also has a deal with Merck & Co. Inc. for Cozaar XQ (launched as Amosartan in Korea), a combination of Pfizer Inc.’s amlodipine (Norvasc) and Merck’s losartan.
“We think the Columbus Project along with other government projects have helped to establish a bridgehead for Korean pharma to move to the North American market, the biggest pharmaceutical market,” the health ministry told PharmAsia News, noting it expects more Korean pharma to advance to the U.S. market in coming years.
Meanwhile, Korean peers are making progress in other developed markets such as Japan and Europe.
LG Life Sciences Ltd. signed a co-development deal in 2012 with Japan’s Mochida Pharmaceutical Co. Ltd. to develop and commercialize antibody biosimilars (Also see "Korea’s LG Life Sciences And Japan’s Mochida Cut Biosimilar Deal" - Scrip, 14 Nov, 2012.).
And, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended marketing authorization June 28 for Celltrion Inc.’s infliximab, which is set to become the first monoclonal antibody biosimilar approved in Europe (Also see "Hospira, Korea’s Celltrion Get EU Nod For First Monoclonal Antibody Biosimilar" - Scrip, 28 Jun, 2013.).