Pharma’s Aim: Tap Innovation In Emerging Markets To Fix Woes At Home (Part 1 of 2)
This article was originally published in PharmAsia News
Executive Summary
Pharma must learn how to apply the best of its experiences in emerging markets to its traditional business, or, experts warn, invigorated competition from more nimble, faster-growing local players that have enjoyed success on their own turf will become a global threat.
Given pharma’s home-market challenges, its foray into emerging markets has so far largely centered on growing its top and bottom lines via old-fashioned clout (a buildup of local sales and marketing, manufacturing, and R&D infrastructure), creative partnering, and adapting to local market needs.
But multinational companies should also seize the opportunity to use emerging markets as laboratories for new business models aimed at improving global market access, health care delivery, and clinical trial efficiency, all areas of key strategic importance to industry.
As industry outsources the machinery of discovery, translational medicine, and clinical development to emerging markets, it’s only a matter of time before innovative assets begin to emerge from these territories.
Sanofi, which derives more than one-third of its sales from emerging markets, has been proactively tapping innovation there and now sees global opportunities for some of its initiatives.
For all its bluster about the various attractions of emerging markets, pharma’s foray into them has thus far been decidedly one dimensional. Multinationals’ current focus on tapping into new territories to juice top-line growth is understandable, especially given the severity of the business challenges on its home turf and the vast potential opportunities in core developing countries. Their efforts to harness innovation in emerging markets, whether scientific or strategic, have been less forceful, however, and remain largely a longer-term goal.
Yet, how Big Pharma perceives and accesses innovation in emerging markets is critically important to its welfare. Emerging markets offer the potential for access to new scientific insights, but they also require different kinds of commercial models and health care delivery to reach vastly heterogeneous patient populations. These strategies, viable opportunities in their own right, can also serve as pilot projects – whether intentionally or not – for new approaches sorely needed in the West.
The global uptake by a multinational company of a novel technology or strategy originally designed to address a specific need in an emerging market is known as reverse innovation. It has been an important strategy in some industries for decades, but, experts say, slow to come to pharma. (Also see "Author/ Professor Chris Trimble Explains Why Reverse Innovation Is “Oxygen,” Not Optional For MNCs: An Interview With PharmAsia News (Part 1 Of 2)" - Scrip, 3 Aug, 2012.) and ( (Also see "Author/ Professor Chris Trimble Explains Why Reverse Innovation Is “Oxygen,” Not Optional For MNCs: An Interview With PharmAsia News (Part 2 Of 2)" - Scrip, 6 Aug, 2012.)).
Still, top strategists at pharma companies see it as part of their armamentarium, useful for responding to Western markets’ growing concern about cost and total care. If pharma continues to rely on its traditional toolkit for addressing competitive pressures, strategists argue, it will end up facing smaller, more entrepreneurial, lower-cost companies from emerging markets. To date, these companies fall under the radar, likely to turn in modest compounded annual growth rate (CAGR) on average of 5 to 10% for the next five to 10 years, says Ajay Dhankhar, a director at McKinsey & Co., but already some, buttressed by stellar domestic performance, have greater ambitions.
Although this train of thinking is finding its way into Big Pharma’s executive suite, it is not always clear whether corporations are systemically capturing such learnings – or even applying them ad hoc.
“I don’t think this issue is hitting health care in the face right now, but unless we recognize this force over the next five to 10 years, it is going to be quite a critical factor,” Dhankhar said during a panel at Elsevier Business Intelligence’s Pharmaceutical Strategic AlliancesConference in September.
And while reverse innovation is a concept that looms on the horizon, not an immediate blueprint for action, it has relevance for biopharma business development executives as they engage increasingly in the formation of non-traditional alliances in emerging markets, with governments, local suppliers, non-profit organizations, and with peers.
Several kinds of emerging markets innovation have potential to help Western companies figure out the optimal responses to challenges they face at home, strategists say. These are interrelated, but for simplicity’s sake can be segmented as follows: new business models related to market access and health care delivery, and scientific or technological developments.
A Global Approach To Local Innovation
Market access may offer the nearest-term upside and therefore be the most likely kind of reverse innovation for MNCs to implement. It is one of the biggest hurdles for pharma companies in emerging markets and an increasing headache in traditional markets as well. Solutions range from the narrow and specific, such as use of information technologies – cell phone apps to communicate medical information inexpensively and routinely to constituents – to more ambitious efforts to make drugs affordable with the least damage to the bottom line.
Interrelated are improvements in R&D productivity and more efficient and effective delivery of care. The former is one of the drivers behind pharma’s massive efforts to externalize a high proportion of its drug development and move clinical trial activities to emerging markets.
The latter is a priority for manufacturers in emerging markets, where health care infrastructure may be limited or non-existent. To be successful, companies must incorporate total care of a patient into their mind-set, not just drug sales – an approach that pharma historically has found difficult to grasp on its home turf and is only starting to embrace elsewhere.
“Companies that succeed in reverse innovating will do anything to solve the large-picture problems, including supply chain or business model innovation,” said Dhankhar. The distinction is important because local innovators that take a holistic approach are already performing better than their Western competitors, particularly in medical devices, he added.
So far, it’s way too soon to determine the payoff from an early embrace of reverse innovation. The hurdles are high against its systemic adoption. Innovation, by its nature, is risky, and fostering reverse innovation as a longer-term strategic play may come at the expense of more concrete, traditional opportunities. This creates a tension for global corporations, especially if they already feel embattled, as do many Big Pharmas. And it is not clear who will spearhead efforts to coordinate within heavily siloed MNCs the different functions needed to make such programs work.
Making Low-Cost Market Access A Priority
Pharma in general has not yet developed enough emerging-market-specific products or business models to make reverse innovation a top priority, and it is largely “just beginning” the evolutionary path toward that goal, says David-Alexandre Gros, MD, chief strategy officer at Sanofi and a member of its executive committee. Gros, a former McKinsey consultant who joined the company in September 2011, points out that the French firm is an exception, in part because it became heavily involved in emerging markets earlier than most of its peers and continues to commit more than the industry average to emerging markets activities.
Despite facing one of the industry’s steepest patent cliffs – or perhaps because of it – the French pharma has seen the contribution from its emerging markets activities expand from 24% of its total sales in 2008 to 32% in the second quarter of 2012. That figure is notably higher than that of its peers (the proportion ranges, with GlaxoSmithKline PLC, widely believed to be second, reporting about 26%). Its emerging markets sales topped €10 billion ($13 billion) for the first time in 2011, and Sanofi is now the No. 1 ranked pharma company in terms of revenue in Brazil and No. 2 in China and Russia – three of the biggest and fastest-growing emerging markets. (See Exhibit 1.)
Exhibit 1
Emerging Markets Contributed Most To Sanofi’s 2011 Sales
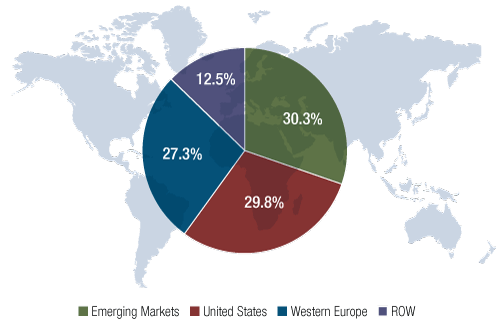
Emerging markets contributed more to Sanofi’s total sales than either Western Europe or the U.S. in 2011. The rest of the world includes Japan, Canada, Australia, and New Zealand.
Sanofi
That growth has come through a mix of dealmaking, savvy commercial moves, and carefully cultivated relations with policy makers, as developing countries overhaul and modernize their regulatory systems and create incentives to encourage local R&D. Even as some of its peers remain heavily dependent on blockbusters, Sanofi has stressed diversification, both geographically and by type of asset (Also see "Sanofi Global Operations President Hanspeter Spek On Building A Diversified Strategy In Emerging Markets: An Interview With PharmAsia News (Part 2 of 2)" - Scrip, 6 Apr, 2012.).
In its diabetes business, Sanofi has moved to a “beyond the molecule” strategy, which integrates its innovative drug business with additional medical offerings, and it is exploring similar options for its other therapeutic franchises (Also see "Biopharmaceuticals Beyond The Drug: Changing Pharma's Value Proposition" - In Vivo, 1 Oct, 2011.).
“We’ve moved [our business focus] from traditional pills with patent risks to products that have high barriers to entry, not just patent protection,” Gros says, adding that the company is looking for innovation “across the value chain around the world, spending time throughout Asia and Latin America.”
Emerging markets is therefore ingrained in Sanofi’s “DNA,” Gros says, enabling the company to act “like a local player,” with products and business models that have been carefully adapted to meet local needs. The company already has taken products and services from emerging markets beyond the markets’ borders.
Still, reverse innovation opportunities are rare, Gros adds. Seizing those opportunities requires a major cultural shift away from, among other things, the blockbuster model. He observes that the French pharma is midway through an evolutionary process that would integrate reverse innovation fully as a strategy.
A Future In Lower-Cost Vaccines
Sanofi’s Shantha Biotechnics Ltd. vaccine business located in Hyderabad, India, offers perhaps the company’s most visible near-term opportunity for reverse innovation (Also see "Off The WHO Hook, Sanofi CEO Viehbacher Plans To Take Shantha's Vaccines To The World" - Scrip, 5 Oct, 2011.). Already a dominant global player in vaccines, Sanofi acquired Shantha in 2009 for more than $700 million in order to gain capabilities in developing, producing, and selling high-quality, affordable vaccines and to expand its biologics production capabilities. Among Shantha’s attractions were a rotavirus vaccine, then in preclinical development, and the potential of inexpensive combination vaccines for emerging markets. The thinking was that Shantha, as a stand-alone subsidiary, would sell the products in emerging markets, while Sanofi could push them out in more traditional venues. [See Deal] (See Exhibit 2.)
Exhibit 2
Since 2008, Sanofi’s Deals Build Leadership In Emerging Markets
|
Parent Or Acquisition Target/Location |
Date |
Type Of Deal |
What Sanofi Got |
|
Genfar/Colombia |
Oct. 2012 |
M&A |
Top spot in Colombian pharma industry and leading South American generics manufacturer, with $133 million in 2011 sales. In addition to generics, Genfar sells animal health and OTC products throughout Central and South America. |
|
Emcure Pharmaceuticals/India |
Feb. 2012 |
Out-license |
Emcure will sell Sanofi-Pasteur’s Verorab anti-rabies vaccine in India. |
|
Universal Medicare/India |
Aug. 2011 |
M&A |
Branded nutraceutical business of privately held nutritional marketing company, bought by Sanofi reportedly for more than $100 million. Purchase is Sanofi’s entry into the Indian OTC market. |
|
Glenmark Pharmaceuticals/India |
May 2011 |
Exclusive in-license |
Exclusive rights to develop and sell Glenmark’s GBR500 monoclonal antibody, in Phase II for Crohn’s disease. |
|
BMP Sunstonep/China |
Oct. 2010 |
M&A |
Full control of Chinese specialty pharmaco for roughly $511 million on a fully diluted basis, or 28% premium per share. |
|
Nepentes/Poland |
May 2010 |
M&A |
Acquisition of Polish OTC company for ~ €105 million. |
|
Glenmark Pharmaceuticals/India |
May 2010 |
Exclusive license |
Exclusive rights in certain countries, including major Western markets and Japan, to Glenmark’s transient receptor potential vanilloid 3 (TRPV3) antagonists for chronic pain. Glenmark gets co-promote rights in the US and several Eastern European markets and exclusive rights in India and other parts of the world. Up-front to Glenmark of $20 million, plus up to $305 million in additional milestones and double-digit royalties on sales. |
|
Minsheng Pharmaceutical Group/China |
Jan. 2010 |
Joint venture |
Consumer health JV focused on vitamin and mineral supplements. |
|
Shantha Biotechnics/India |
July 2009 |
M&A |
Vaccine business owned by the Merieux Alliance and ShanH bought by Sanofi for €550 million (>$700 million). |
|
Kendrick Farmaceutica/Mexico |
April 2009 |
M&A |
Acquisition of Mexican generics maker for undisclosed sum. Kendrick holds about 15% share of domestic market in generics. |
|
Medley Pharmaceuticals/ Brazil |
April 2009 |
M&A |
Acquisition of leading Brazilian generics & OTC manufacturer for €500 million. Solidifies Sanofi’s position as leader in Latin American generics. |
|
Zentiva BV/Czech Republic |
June 2008 |
M&A |
Acquisition of shares of leading Czech generics maker that Sanofi does not already own for about $1.9 billion. |
Source: Elsevier’s Strategic Transactions
Shantha, which was founded in 1993 and grew to become one of India’s largest vaccine makers, already had a small portfolio of vaccines, which it sold in developing countries. These include a recombinant DNA hepatitis B vaccine and the oral cholera vaccines, Shanchol, which is pre-qualified by the World Health Organization – a designation that implies high quality and is an endorsement necessary to public health organizations and governments that buy the bulk of vaccines for emerging markets countries.
In 2010, Shantha had quality issues with production of its popular Shan5 combination pentavalent vaccine, (diphtheria, tetanus, pertussis, hepatitis B, and haemophilus influenza B), which led WHO to delist the product. Sanofi says those problems are fixed and it hopes to re-launch the vaccine in time for the 2013–2016 UNICEF tenders, but the event highlights in general that much risk remains around quality in emerging markets. (See (Also see "WHO Does What It Warned: Delists Shantha Biotech's Pentavalent Vaccine Shan5; More Pains For Sanofi-Aventis?" - Scrip, 3 Aug, 2010.).) In addition, Shantha’s pipeline includes Shan6, a preclinical hexavalent vaccine, which adds inactivated polio to Shan5’s mix; a rotavirus vaccine in Phase I; and a novel human papilloma virus vaccine also in preclinical development, all aimed at much lower price points than vaccines sold in the West.
The plan is for Shantha to sell the vaccines to government and quasi-government agencies throughout the developing world. Sanofi already makes some of the vaccines Shantha is developing and could also incorporate some of Shantha’s portfolio into its own commercial network geared toward more traditional and middle-income markets.
“It’s a key question for Sanofi,” says Gros, referring to “process innovation” that allows a manufacturer to produce a vaccine in emerging markets at a fraction of its current cost. The pressure is mounting to master that capability because Chinese vaccine exporters, although still much smaller in terms of aggregate sales, are on the verge of becoming competitive threats, with impressive economies of scale. Their status improved in 2011 after WHO approved for the first time China’s official vaccine production standards, a key step toward obtaining WHO pre-certification (Also see "Chinese Manufacturers Will Cut Into India's Vaccine Exports Unless Indian Firms Can Find New Approach - World Vaccine Congress Asia" - Scrip, 29 Jun, 2011.) and (Also see "Are China’s Vaccines Ready For The World?" - Scrip, 12 Jan, 2012.).
[Editor’s note: This story also appeared in IN VIVO![]() , October 2012; part two will appear in an upcoming issue of PharmAsia News.]
, October 2012; part two will appear in an upcoming issue of PharmAsia News.]