INFOGRAPHIC: Regeneron's 2Q Eylea gains, pipeline catalysts
This article was originally published in Scrip
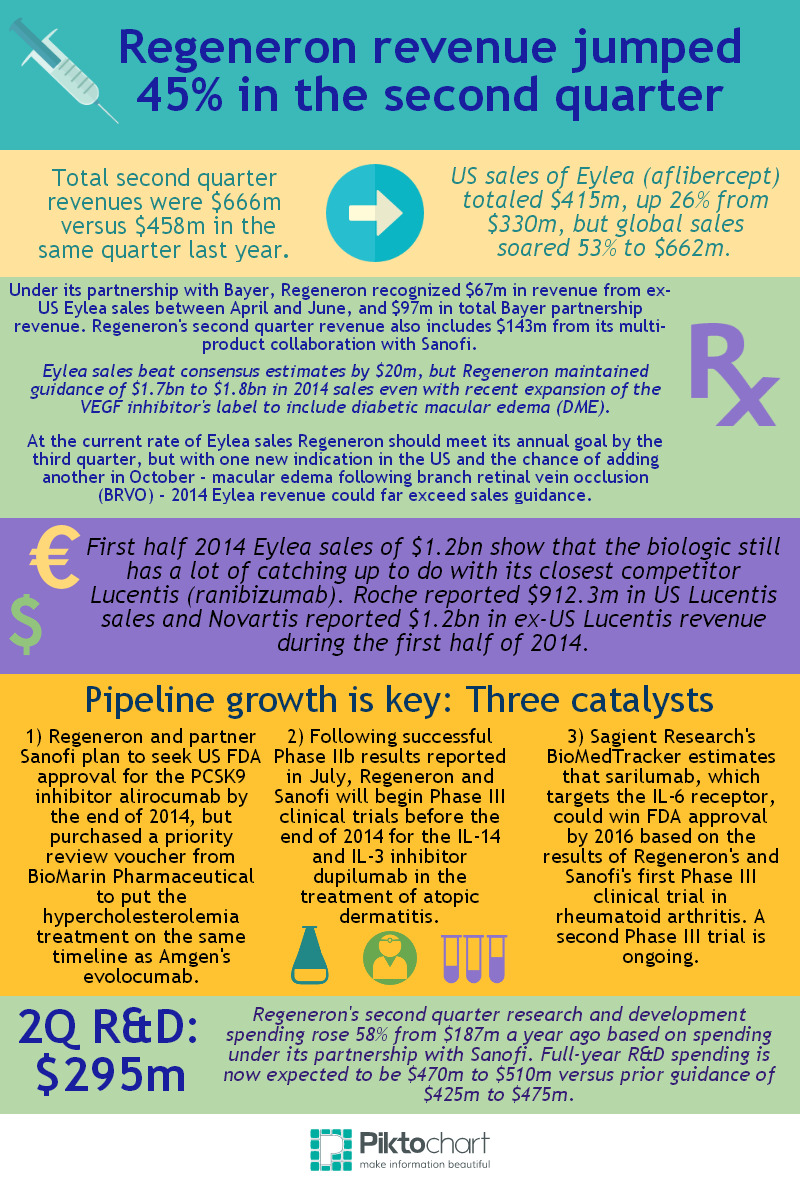
Regeneron Pharmaceuticals is riding high based on the Tarrytown, New York-based company's 45% rise in second quarter revenue to $666m versus the same period last year and a 53% jump in global Eylea (aflibercept) sales to $662m.
Regeneron's stock gained $7.76 per share, or 2.4%, to close at $333.20 on 5 August when the second quarter earnings were announced. The stock rose another 2.3% to close at $340.69 on 6 August, bringing the share price closer to its one-year high of $352.49.
Eylea has at least two opportunities for growth in the second half of 2014 after recent US FDA approval of the biologic as a treatment for diabetic macular edema (DME) (scripintelligence.com, 30 July 2014). FDA approval in macular edema following branch retinal vein occlusion (BRVO) is expected in October.
"Regeneron's sales force has already begun calling on the physicians who treat DME and are largely the same physicians that treat wet age-related macular degeneration (AMD). A billing code for Eylea in DME is already in place and Regeneron noted that it will benefit from significant operating leverage with its already-in-place Eylea reps," Leerink Swann analyst Joseph Schwartz wrote in a 5 August research note.