Iclusig gets EU reprieve but with 'increased caution'
This article was originally published in Scrip
The EMA's Pharmacovigilance Risk Assessment Committee (PRAC) has said doctors should continue to use Ariad Pharmaceuticals' leukemia therapy Iclusig – despite sales of the drug being suspended by the company in the US amid safety concerns.
On 31 October Ariad temporarily suspended sales of Iclusig (ponatinib), at the US FDA's request following reports of serious and life-threatening blood clots and severe narrowing of blood arteries and veins in patients taking the medicine (scripintelligence.com, 1 November 2013).
The FDA also advised that US patients currently taking Iclusig who are not responding to the drug should immediately stop taking the medicine and discuss alternative treatment options with their healthcare professionals. However the regulator said that patients taking Iclusig who are responding to the drug, and whose healthcare professionals determine the potential benefits outweigh the risks, should be treated under a single-patient investigational new drug application (IND) or expanded access registry program while the FDA's safety investigation continues (scripintelligence.com, 1 November 2013). Furthermore, Ariad terminated a study of Iclusig in newly diagnosed patients in October because of concerns of life-threatening blood clots severe narrowing of blood arteries and veins in patients taking the drug (scripintelligence.com, 21 October 2013).
However CEO Harvey Burger said at the time of the US suspension that it was "too early to know exactly how the EMA and the European countries will react to our decision to suspend marketing in the US."
The PRAC has now responded with its advice for the EMA, following its own investigation of the oral leukemia drug, saying that "conditions related to thrombosis such as myocardial infarction (heart attack) are known side-effects of Iclusig and the current EU product information mentions the risk of myocardial infarction, cerebral infarction (stroke) and related disorders."
The PRAC found that the benefits of the anticancer medicine outweigh the risks for patients. The committee has advised that patients and healthcare professionals can continue to use the Iclusig with increased caution in its authorized use. However, patients should be monitored carefully for evidence of thromboembolism and vascular occlusion. The PRAC also concluded that Iclusig's product information should be updated to include strengthened warnings on the cardiovascular risk. In addition to the warning update the PRAC said there is still a need to carry out "an in-depth review of the medicine's benefit-risk profile."
The CHMP is expected to issue an opinion on the PRACs findings during its next meeting, which will take place from 18 to 21 November.
Massachusetts-based Ariad's stock (Nasdaq) was up 1.75% in mid-afternoon trading on the 8 November following the PRAC's report. The company's stock has tumbled dramatically since the start of October, falling from $18.40 on 30 September to a low of $2.20 on 31 October.
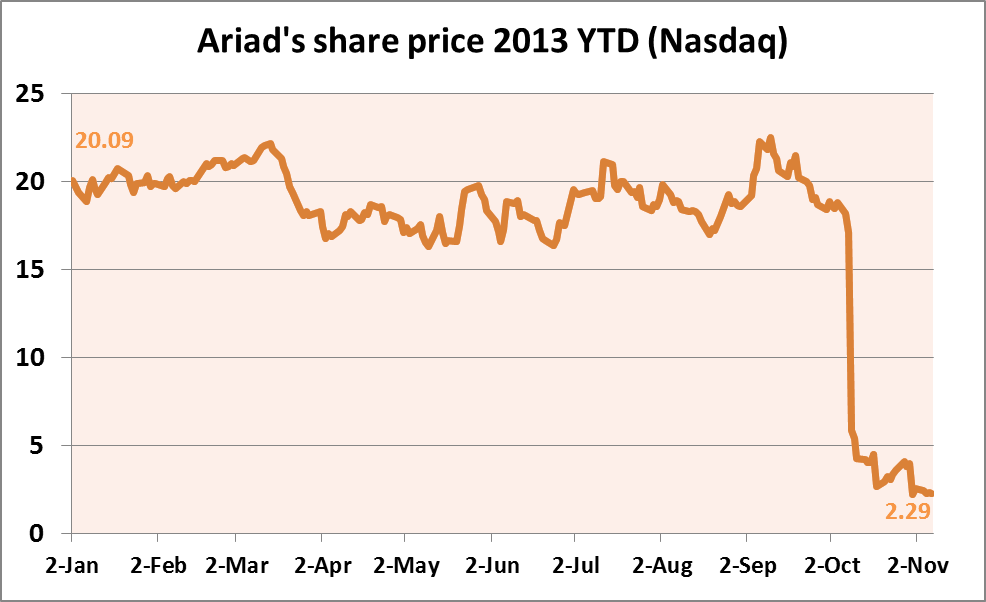
Source: Yahoo Finance
Shares of the company dropped more than 40% on the 21 October alone when the company revealed it would terminate its study of Iclusig in newly diagnosed leukemia patients (scripinteligence.com, 21 October 2013). Ariad's current market cap is $431.3m.
Inclusig, an anticancer medicine belonging to the class of tyrosine kinase inhibitors, is indicated in Europe as a treatment for adult patients with:
- chronic phase, accelerated phase or blast phase chronic myeloid leukemia (CML) who are resistant to dasatinib or nilotinib; who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation;
- Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) who are resistant to dasatinib; who are intolerant to dasatinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation
