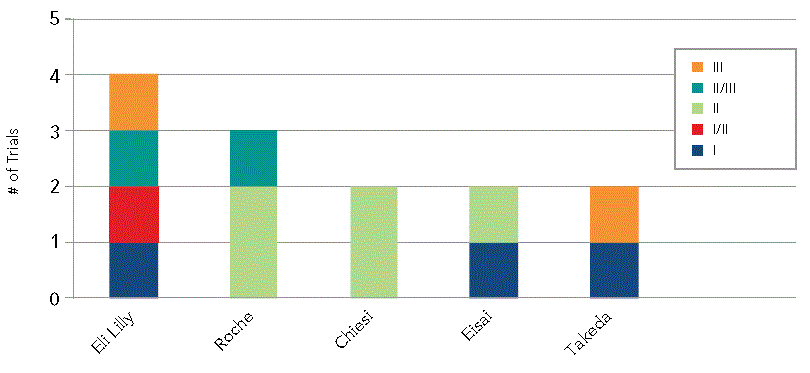Lilly leads big pharma in new Alzheimer's paradigm
This article was originally published in Scrip
Lilly is the leading big pharma player in a new paradigm of Alzheimer's disease drug development, according to the latest analysis by Citeline. The firm is conducting four clinical trials (from Phase I to Phase III) in earlier stage patients, making it the top trial sponsor in the emerging concept of targeting treatment at patients with mild cognitive impairment with a view to preventing progression to AD.
This particular approach has also been taken up by Roche (overall the current top sponsor of AD trials with 24 ongoing studies in the clinic), Chiesi, Eisai and Takeda, as well as a larger number of smaller pharmaceutical companies.
Top 5 Sponsors in New Paradigm
Source: Trialtrove, 2013 Citeline
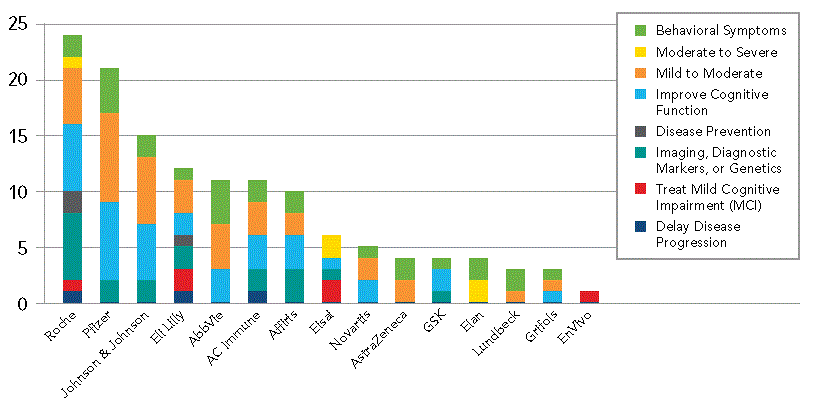
However, several big pharma players that are traditionally very active in AD drug development have not yet focused in on this new paradigm, including Pfizer, Johnson & Johnson, Novartis and AbbVie. It is therefore likely that these smaller companies are likely to be much in demand for partnering and/or acquisition deals by big pharma players should their trials progress successfully.
Companies in this category at the Phase II stage include QR Pharma, Prana biotechnology, BioArtic Neuroscience, and Chiesi itself.
Top 15 Sponsors by Patient Segments in Ongoing and Planned Trials, Phase I-III
Source: Trialtrove, 2013 Citeline
One factor that may be holding big pharma firms back at present – aside from the high risk associated with treating patients with few or no symptoms – is the fact that they "are likely awaiting guidelines from the FDA on how to design trials", commented Citeline analysts Dr Laura Withington and Deborah Jeanfavre in the report, entitled Alzheimer's New Paradigm: Hit the Target before the Symptoms.
Meanwhile, the authors note that further research and development of novel biomarkers to identify patients and to correlate treatment with efficacy is "greatly needed".
Secretase inhibition and targeting beta amyloid production, accumulation or clearance are by far the predominant approaches in development, accounting for 48% of all trials in AD and an even starker 70% of ongoing trials specifically treating early-stage Alzheimer's disease. These two mechanisms are part of the same pathway, and contrast with the current therapies on the market, Pfizer/Eisai's Aricept (donepezil), Novartis's Exelon (rivastigmine) and Johnson & Johnson's Razadyne (galantamine), which are all acetylcholinesterase (AChe) inhibitors.
Important clinical trial results that will help establish the efficacy of targeting the amyloid pathway at an earlier stage of the disease should be reported within the next year. Roche and Lilly are both expected to report Phase III results (with gantenerumab and solanezumab) by July 2014, while Chiesi is expected to report Phase II efficacy results for CHF-5074 in late 2013.
The majority of current Phase II and Phase III trials are expected to be completed, however, in 2016, by which time there should have been time also to advance in the correlation of biomarkers with efficacy in early-stage patients.
"In three years' time, those invested may have some idea whether treating this early stage population is viable, but whether big pharma or small pharma is the one to gain is a toss of the coin," the report concluded.
Citeline's report is available as a free download here .